Pople diagram

A Pople diagram or Pople's Diagram is a diagram which describes the relationship between various calculation methods. It was initially introduced in January 1965 by Sir John Anthony Pople, KBE FRS (31 October 1925 – 15 March 2004), during the Symposium of Atomic and Molecular Quantum Theory in Florida.[1] The Pople Diagram can be either 2-dimension or 3-dimensional, with the axes representing Ab initio quantum chemistry methods, Basis sets used in computational chemistry and treatment of relativity.[2] The diagram attempts to balance calculations by giving all aspects of a computation equal weight.
History

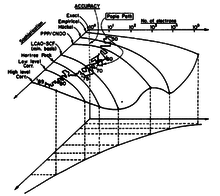
John Pople first introduced the Pople Diagram during the Symposium on Atomic and Molecular Quantum Theory held on Sanibel Island, Florida, in January 1965. He called it a "hyperbola of quantum chemistry", which illustrates the inverse relationship between the sophistication of a calculational method and the number of electrons in a molecule that can be studied by that method.[1] Alternative (reverse) arrangement of the vertical axis or interchange of the two axes are also possible.[3][4]
Three-Dimensional Pople Diagrams
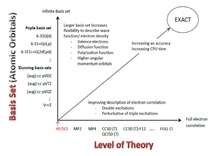
The 2-dimension Pople diagram describes the basis set and computational methods used in computations. The higher the value of the graph shows higher accuracy, sophistication as well as overall computational costs.[2] Since the computational Basis set can potentially have infinite sophistication, it will be impossible for quantum chemists to find exact solutions for their calculations. Thus, all calculations done using computational methods are mere approximations.
See also
- John Pople
- Computational Chemistry
- Basis set (chemistry)
- Ab initio quantum chemistry methods
- Electronic correlation
References
- 1 2 Karplus, Martin (July 1990). "Three-dimensional "Pople diagram"". J. Phys. Chem. (ACS.) 14 (94): 5435–5436. doi:10.1021/j100377a002. Retrieved October 21, 2015.
- 1 2 Dolg, Michael (17 February 2015). Computational Methods in Lanthanide and Actinide Chemistry. John Wiley & Sons, 2015. p. 480. ISBN 9781118688281.
- ↑ Vereecken, Luc; Franciscob, Joseph (2012). "Theoretical studies of atmospheric reaction mechanisms in the troposphere". Chem. Soc. Rev. 41: 6259–6293. doi:10.1039/C2CS35070J.
- ↑ Auer, Alexander A. (September 4, 2014). "Electron Correlation - Methods beyond Hartree-Fock, how to approach chemical accuracy" (PDF). Max-Planck-Institute for Chemical Energy Conversion, Mülheim (MMER Summerschool 2014 - Electron Correlation). Retrieved October 21, 2015.