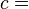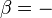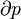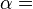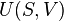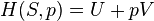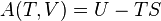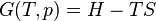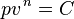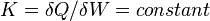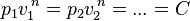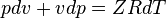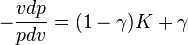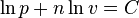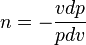Polytropic process
| Thermodynamics | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
The classical Carnot heat engine | ||||||||||||
|
Branches |
||||||||||||
|
||||||||||||
| Book:Thermodynamics | ||||||||||||
A polytropic process is a thermodynamic process that obeys the relation:
where p is the pressure, v is specific volume, n is the polytropic index (any real number), and C is a constant. All processes that can be expressed as a pressure and volume product are polytropic processes. Some of those processes (n=0,1,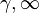 ), are unique. This equation can accurately characterize a very wide range of thermodynamic processes, that range from n=0 to n=
), are unique. This equation can accurately characterize a very wide range of thermodynamic processes, that range from n=0 to n=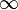 which covers, n=0 (isobaric), n=1 (isothermal), n=γ (isentropic), n=
which covers, n=0 (isobaric), n=1 (isothermal), n=γ (isentropic), n=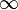 (isochoric) processes and all values of n in between. Hence the equation is polytropic in the sense that it describes many lines or many processes. In addition to the behavior of gases, it can in some cases represent some liquids and solids. The polytropic process equation is particularly useful for characterizing expansion and compression processes which include heat transfer. The one restriction is that the process should display a constant energy transfer ratio K during that process:
(isochoric) processes and all values of n in between. Hence the equation is polytropic in the sense that it describes many lines or many processes. In addition to the behavior of gases, it can in some cases represent some liquids and solids. The polytropic process equation is particularly useful for characterizing expansion and compression processes which include heat transfer. The one restriction is that the process should display a constant energy transfer ratio K during that process:
If it deviates from that restriction it suggests the exponent is not a constant.
For a particular exponent, other points along the curve that describes that thermodynamic process can be calculated:
Derivation

The following derivation is taken from Christians.[1] Consider a gas in a closed system undergoing an internally reversible process with negligible changes in kinetic and potential energy. The First Law of Thermodynamics states that the energy added to a system as heat, minus the energy that leaves the system as work, is equal to the change in the internal energy of the system:
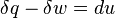 (Eq. 1)
(Eq. 1)
Energy entering the system increases the energy of the system, and energy leaving the system decreases the energy of the system. The sign convention is that heat transfer into the system is positive. Work done by the system is also positive. With this sign convention, the heat transfer term is added to 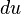 , and the work term is subtracted from
, and the work term is subtracted from 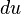 .
.
Define the energy transfer ratio,
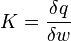 or
or
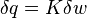 .
.
For an internally reversible process the only type of work interaction is moving boundary work, given by 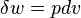 .
.
By substituting the above expressions for  , and
, and  into The First Law it can then be written
into The First Law it can then be written
 (Eq. 1)
(Eq. 1)
Consider the Ideal Gas equation of state with the well-known compressibility factor, Z: pv = ZRT. Assume the compressibility factor is constant for the process. Assume the gas constant is also fixed (i.e. no chemical reactions are occurring, hence R is constant). The pv = ZRT equation of state can be differentiated to give
Based on the well-known specific heat relationship arising from the definition of enthalpy, the term ZR can be replaced by cp - cv. With these observations the First Law (Eq. 1) becomes
where γ is the ratio of specific heats cp/cv. This equation will be important for understanding the basis of the polytropic process equation. Now consider the polytropic process equation itself:
Taking the natural log of both sides (recognizing that the exponent n is constant for a polytropic process) gives
which can be differentiated and re-arranged to give
By comparing this result to the result obtained from the First Law, it is concluded that when the energy transfer ratio is constant for the process, the polytropic exponent is a constant and therefore the process is polytropic. In fact the polytropic exponent can be expressed in terms of the energy transfer ratio:
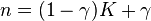 .
.
where the term  is negative for an ideal gas.
is negative for an ideal gas.
This derivation can be expanded to include polytropic processes in open systems, including instances where the kinetic energy (i.e. Mach Number) is significant. It can also be expanded to include irreversible polytropic processes (see Ref [1]).
Applicability
The polytropic process equation is usually applicable for reversible or irreversible processes of ideal or near-ideal gases involving heat transfer and/or work interactions when the energy transfer ratio δq/δw is constant for the process. The equation may not be applicable for processes in an open system if the kinetic energy (i.e. Mach Number) is significant. The polytropic process equation may also be applicable in some cases to processes involving liquids, or even solids.
Polytropic Specific Heat Capacity
It is denoted by  and it is equal to
and it is equal to
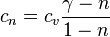
Relationship to ideal processes
For certain values of the polytropic index, the process will be synonymous with other common processes. Some examples of the effects of varying index values are given in the table.
| Polytropic index |
Relation | Effects |
|---|---|---|
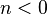 |
— | Negative exponents reflect a process where the amount of heat being added is large compared to the amount of work being done (i.e. the energy transfer ratio > γ/(γ-1)). Negative exponents can also be meaningful in some special cases not dominated by thermal interactions, such as in the processes of certain plasmas in astrophysics.[2] |
 |
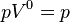 (constant) |
Equivalent to an isobaric process (constant pressure) |
 |
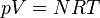 (constant) |
Equivalent to an isothermal process (constant temperature) |
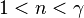 |
— | A quasi-adiabatic process in which the heat flow and work flow are in opposite directions (positive K), such as in vapor compression refrigeration during compression, where the elevated vapour temperature resulting from the work done by the compressor on the vapour leads to some heat loss from the vapour to the cooler surroundings. Also a "polytropic compression" process like gas through a centrifugal compressor where heat loss from the compressor (into environment) is greater than the heat added to the gas through compression. |
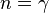 |
— | 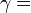 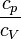 is the isentropic exponent, yielding an isentropic process (adiabatic and reversible). It is also widely referred as adiabatic index, yielding an adiabatic process (no heat transferred). However the term adiabatic does not adequately describe this process, since it only implies no heat transfer.[3] Only a reversible adiabatic process is an isentropic process. is the isentropic exponent, yielding an isentropic process (adiabatic and reversible). It is also widely referred as adiabatic index, yielding an adiabatic process (no heat transferred). However the term adiabatic does not adequately describe this process, since it only implies no heat transfer.[3] Only a reversible adiabatic process is an isentropic process. |
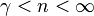 |
— | Normally polytropic index is greater than specific heat ratio (γ) within a "polytropic compression" process like gas through a centrifugal compressor. The inefficiencies of centrifugal compression and heat added to the gas outweigh the loss of heat into the environment. Also a quasi-adiabatic process in which the heat flow and work flow are in the same direction (negative K), such as in an internal combustion engine during the power stroke, where heat is lost from the hot combustion products, through the cylinder walls, to the cooler surroundings, at the same time as those hot combustion products do work on the piston. |
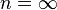 |
— | Equivalent to an isochoric process (constant volume) |
When the index n is between any two of the former values (0, 1, γ, or ∞), it means that the polytropic curve will be bounded by the curves of the two corresponding indices.
Note that 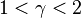 , since
, since 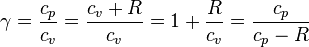 .
.
Notation
In the case of an isentropic ideal gas, 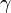 is the ratio of specific heats, known as the adiabatic index or as adiabatic exponent.
is the ratio of specific heats, known as the adiabatic index or as adiabatic exponent.
An isothermal ideal gas is also a polytropic gas. Here, the polytropic index is equal to one, and differs from the adiabatic index 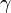 .
.
In order to discriminate between the two gammas, the polytropic gamma is sometimes capitalized, 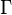 .
.
To confuse matters further, some authors refer to 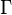 as the polytropic index, rather than
as the polytropic index, rather than  . Note that
. Note that
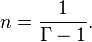
Other
A solution to the Lane-Emden equation using a polytropic fluid is known as a polytrope.
See also
- Polytrope
- Adiabatic process
- Isentropic process
- Isobaric process
- Isochoric process
- Isothermal process
- Vapor compression refrigeration
- Gas compressor
- Internal combustion engine
- Quasistatic equilibrium
- Thermodynamics
References
- 1 2 Christians, Joseph, "Approach for Teaching Polytropic Processes Based on the Energy Transfer Ratio, International Journal of Mechanical Engineering Education, Volume 40, Number 1 (January 2012), Manchester University Press
- ↑ G. P. Horedt Polytropes: Applications In Astrophysics And Related Fields, Springer, 10/08/2004, pp.24.
- ↑ GPSA book section 13