Polyphthalamide

Polyphthalamide (aka. PPA, High Performance Polyamide) is a diverse subset of thermoplastic synthetic resins in the polyamide (nylon) family defined as when 60% or more moles of the carboxylic acid portion of the repeating unit in the polymer chain is composed of a combination of terephthalic (TPA) and isophthalic (IPA) acids.[1] Some of them have been used to replace metals in high temperature automotive applications, as the housing for high temperature electrical connectors and multiple other uses. PPAs are polyamides containing aromatic rings in the diacid part of their backbones, which gives them high mechanical strength and thermal resistance.[2]
As a member of the nylon family, it is a semi-crystalline or amorphous material composed from a diacid and a diamine which means PPA is formed by the reaction of aromatic acids with aliphatic diamines. However, the diacid portion contains at least 55% terephthalic acid (TPA) or isophthalic acid (IPA).[3] TPA or IPA are aromatic components which serve to raise the melting point, glass transition temperature and generally improve chemical resistance vs. standard aliphatic nylon polymers and reduce water absorption.[4] If more than 55% of the acid part of a PPA is made out of IPA, then the copolymer is amorphous.[5]
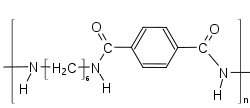 Polyphthalamide with 6T-Segment
Polyphthalamide with 6T-Segment
 Polyphthalamide with DT-Segment
Polyphthalamide with DT-Segment

In recent years, polyphthalamides have been commercialized by several polymer resin manufacturers which are under the trade names Amodel, Grivory, Ultramid T, VESTAMID HTplus, Akromid T and Zytel HTN.
Properties
Properties of polyphthalamide include: high heat resistance, high chemical resistance, abrasion/corrosion resistance, high tensile strength, high dimensional stability, direct bonding to many elastomers to give plastic-rubber composites, and is also approved for direct contact with drinking water and food.[6] PPA is more creep resistant and stiff compared to traditional polyamide nylons. Molar masses for PPA’s made with direct polycondensation techniques range between 12,000 and 16,000 g/mol. The glass transition temperature of PPA increases as the amount of TPA increases.[7] The challenge facing polymer design compared to metal design is the fact that the mechanical properties of polymeric materials are much more time and temperature dependent. While polymers generally do not corrode, their properties may be negatively affected by environmental factors such as ultraviolet radiation, humidity, and exposure to chemicals.[8] PPA resin technology can produce both semi-crystalline and amorphous polymers. One of the major differences between amorphous and semi-crystalline polymers is the way their properties change in response to changes in temperature. At temperatures above the glass transition temperature the modulus decreases rapidly. Therefore, amorphous thermoplastics are rarely used at temperatures higher than their glass transition temperature.[9]
Like aliphatic nylons, PPAs can be modified with reinforcing agents, toughens stabilizers etc. for example 51% Glass/Mineral reinforced PTA is added by weight. Its flexural strength is 24 to 28.4 GPa. Its elongation at break is 1.9 to 2%, and specific gravity is 1.66 to 1.68.[10]
Polyphthalamide Blends
In the article Characterization and properties of polyphthalamide/polyamide blends and polyphthalamide/polyamide/polyolefin blends the author Desio found that in general, PA addition to PPA (PPA/PA blend) lowers the melting point and glass transition temperature, which potentially makes these polyphthalamide blends easier to process when compared to higher melting/softening PPAs.
While there have been large investigations into PA/polyolefin blends, little is known about the properties of PPA/ polyolefin blends. Seemingly, due to the fact that extremely high temperatures are required for processing PPA materials.PPA/PA/polyolefin blends exhibit a good balance of ductility, strength, stiffness, impact, and thermal performance, indicating that these types of materials should have commercial utility.[11]
Applications
Polyphthalamide is widely applicable. Automotive uses include fuel and coolant lines, LED headlights, and weight reduction. Medical uses include tubing for devices such as catheters. Electronic applications are LEDs and cable/wire protection. PPA is used in gas pipes and supply lines, in the oil industry, due to its ability to withstand high pressures. Also used in personal care, polyphthalamide is used as toothbrush bristles as well as hairbrushes. Polyphthalamides are even used in sports equipment. A major benefit to polyphthalamide is its suitability for metal substitution applications. One such application is the manufacturing of electronic components via soldering process without lead, which is utilized in the smart phone industry.[12] The automotive and electrical industries use PPA as electrical insulation, switches, and connectors. PPA is also used as motor insulators, pump wear rings, automotive motor bobbin parts, fuel line connectors, water heater manifolds, valve bodies for showers, fuel modules, fuel cut-off valves, thermostat housing, air coolers, coolant pumps, bushings and bearing pads in aircraft engines.
Recycling and Recovery
Another advantage is that PPA is fully recyclable, either mechanically or as raw material. The PPA waste that produces energy can be recovered at incineration plants. The best recovery options depend on many conditions such as local legislation, plastic part design, access to sorting facilities, and recycling costs.
Major suppliers
- Evonik under the brand VESTAMID HT 'plus'
- DuPont under the brand Zytel HTN
- EMS under the brand Grivory. GV grades are based on PA 66/6I/6T. HT1 grades on 6T/6I and HT2 grades on 6T66 [13] and HT3/HT3-CO on copolymers of 10T [14]
- Solvay under the brand AMODEL. Initially commercialized by Amoco, today this brand is owned by Solvay. According to Nevicolor, all current Amodel grades are based on a single polymer, A1100 [15] but there are grades based on 66/6T copolymer.[16] and others on 66/6T/6I copolymer[17]
- BASF under the brand Ultramid T with 6T/6 copolymer.
References
- ↑ Cousin, Thibault, Jocelyne Galy, and Jerome Dupuy. "Molecular Modelling of Polyphthalamides Thermal Properties: Comparison between Modelling and Experimental Results." Elsevier 53.15 (2012): 3203-210. Web. 26 Nov. 2013. <https://wisconsin.hosts.atlas-sys.com/nonshib/GZE/illiad.dll?Action=10&Form=75&Value=1964134>
- ↑ Cousin, Thibault, Jocelyne Galy, and Jerome Dupuy. "Molecular Modelling of Polyphthalamides Thermal Properties: Comparison between Modelling and Experimental Results." Elsevier 53.15 (2012): 3203-210. Web. 26 Nov. 2013.<https://wisconsin.hosts.atlas-sys.com/nonshib/GZE/illiad.dll?Action=10&Form=75&Value=1964134>
- ↑ Lyons, J. S., Tinio, E. G. and Berry, S. F. (1995), Creep, stress rupture, and isothermal aging of reinforced polyphthalamide. J. Appl. Polym. Sci., 56: 1169–1177. doi: 10.1002/app.1995.07056091
- ↑ Harper, Charles A (2002-06-10). Handbook of plastics, elastomers, and composites. pp. 51–52. ISBN 978-0-07-138476-6.
- ↑ Cousin, Thibault, Jocelyne Galy, and Jerome Dupuy. "Molecular Modelling of Polyphthalamides Thermal Properties: Comparison between Modelling and Experimental Results." Elsevier 53.15 (2012): 3203-210. Web. 26 Nov. 2013. <https://wisconsin.hosts.atlas-sys.com/nonshib/GZE/illiad.dll?Action=10&Form=75&Value=1964134>
- ↑ Evonik Industries, http://www.vestamid.com/product/vestamid/en/products-services/pages/default.aspx
- ↑ Cousin, Thibault, Jocelyne Galy, and Jerome Dupuy. "Molecular Modelling of Polyphthalamides Thermal Properties: Comparison between Modelling and Experimental Results." Elsevier 53.15 (2012): 3203-210. Web. 26 Nov. 2013. <https://wisconsin.hosts.atlas-sys.com/nonshib/GZE/illiad.dll?Action=10&Form=75&Value=1964134>
- ↑ http://www.nevicolor.it/images/commercializzati/pdf/amodel/amodel_general_brochure.pdf
- ↑ http://www.nevicolor.it/images/commercializzati/pdf/amodel/amodel_general_brochure.pdf
- ↑ http://www.efunda.com/glossary/materials/polymers/polyphthalamide--51pct_glass_slash_mineral_reinforced--401.cfm
- ↑ Desio, G. P. (1996), Characterization and properties of polyphthalamide/polyamide blends and polyphthalamide/polyamide/polyolefin blends. J Vinyl Addit Technol, 2: 229–234. doi: 10.1002/vnl.10131 http://onlinelibrary.wiley.com/doi/10.1002/vnl.10131/pdf
- ↑ Evonik Industries, http://www.vestamid.com/product/vestamid/en/products-services/pages/default.aspx
- ↑ Erteco. "Metal Replacement" (PDF). Staal Centrum. Staal Centrum. pp. Slide 9. Retrieved 25 May 2015.
- ↑ "Grivory HT". www.emsgrivory.com. EMS Chimie. Retrieved 25 May 2015.
- ↑ "Amodel design guide" (PDF). Nevicolor. p. 13. Retrieved 25 May 2015.
- ↑ "Technical data sheet". IDES. Retrieved 25 May 2015.
- ↑ "Amodel A1133 datasheet". IDES. Retrieved 25 May 2015.