Polyoxometalate
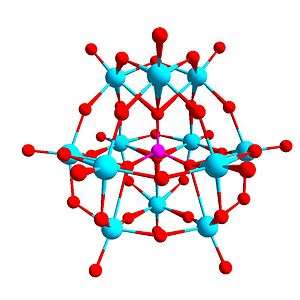
In chemistry, a polyoxometalate (abbreviated POM) is a polyatomic ion, usually an anion, that consists of three or more transition metal oxyanions linked together by shared oxygen atoms to form a large, closed 3-dimensional framework.
The metal atoms are usually group 5 or group 6 transition metals in their high oxidation states. In this state, their electron configuration is d0 or d1. Examples include vanadium(V), niobium(V), tantalum(V), molybdenum(VI), and tungsten(VI).
The framework of transition metal oxyanions may enclose one or more hetero atoms such as phosphorus or silicon, themselves sharing neighbouring oxygen atoms with the framework. For example, the phosphotungstate anion [PW12O40]3− consists of a framework of twelve octahedral tungsten oxyanions surrounding a central phosphate group.
History
The first example of a polyoxometalate compound was ammonium phosphomolybdate, containing the [PMo12O40]3− anion, discovered in 1826.[1] This anion has the same structure as the phosphotungstate anion, whose structure was determined in 1934. This structure is called the Keggin structure after its discoverer.[2] Following this discovery, other fundamental structures such as the Wells-Dawson ion were found, and their chemistry and applications as catalysts were determined.
Polyoxomolybdates include the wheel-shaped molybdenum blue anions and spherical keplerates. Numerous hybrid organic/inorganic materials that contain POM cores,[3][4] new potential applications based on unusual magnetic[5] and optical[6] properties of some POMs, and potential medical applications such anti-tumor and anti-viral uses.
Structure
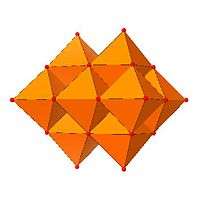
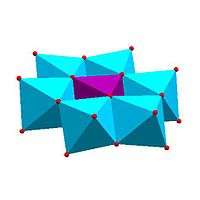
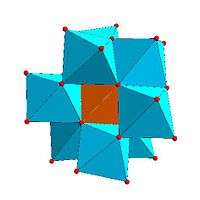
Certain structural motifs recur. The Keggin ion for example is common to both molybdates and tungstates with different central hetero atoms. Examples of some fundamental polyoxometalate structures are shown below. The Lindqvist ion is an iso-polyoxometalate, the other three are hetero-polyoxometalates. The Keggin and Dawson structures have tetrahedrally coordinated hetero-atoms, such as P or Si, and the Anderson structure has an octahedral central atom, such as aluminium.
 |
 |
 |
 |
| Lindqvist hexamolybdate, Mo6O192− | Decavanadate, V10O286− | Paratungstate B, H2M12O4210− | Mo36-polymolybdate, Mo36O112(H2O)168− |
 |
 |
 | |
| Strandberg structure, HP2Mo5O234− | Keggin structure, XM12O40n− | Dawson structure, X2M18O62n− | |
 |
 |
 |
 |
| Anderson structure, XM6O24n− | Allman-Waugh structure, XM9O32n− | Weakley-Yamase structure, XM10O36n− | Dexter-Silverton structure, XM12O42n− |
Framework
The terminal oxide centers of polyoxometallate framework can in certain cases be replaced with other ligands, as S2-, bromide, and NR2-.[1][7] Sulfur-substituted POMs are called a polyoxothiometalate. Other ligands replacing the oxide ions have also been attested, such as nitrosyl and alkoxy groups.[8][9]
The typical framework building blocks are polyhedral units, with 4, 5, 6 or 7 coordinate metal centres. These units share edges and/or vertices, or, less commonly, faces (such as in the ion CeMo12O428−, which has face-shared octahedra with Mo atoms at the vertices of an icosahedron[10]).
The most common unit for polymolybdates is the octahedral MoO6 unit, often distorted by the Mo atom being off-centre to give one shorter Mo-O bond. Some polymolybdates contain pentagonal bipyramidal units; these are the key building blocks in the molybdenum blues.
Hetero atoms

Hetero atoms are present in many polyoxometalates. Many different elements can serve as heteroatoms, with various coordination numbers:
- 4-coordinate (tetrahedral) in Keggin, Dawson, and Lindqvist structures (e. g., PO43-, SiO44-, AsO43-)
- 6-coordinate (octahedral) in Anderson structure (e. g., Al(OH)6, TeO6)
- 8-coordinate (square antiprism) in ((CeO8)W10O28)8−
- 12-coordinate (icosahedral) in (UO12)Mo12O30 8−
The hetero atom may be located in the centre of the anion, as in the Keggin structure, or in the center of a structural fragment, such as the two phosphorus atoms in the Dawson ion, which are central to its two symmetric fragments.
Polyoxometalates bear similarities to clathrate structures. The Keggin ion, for example, could be formulated as PO3−
4@M
12O
36, and the Dawson as (XO2−
4)
2@M
18O
54. The @ notation denotes the physical enclosure of the left-hand side in the right-hand side. Unlike clathrates however, the guest anions cannot be reversibly removed.
Some cage structures that contain other ions are known. For example, the vanadate cage V18O42 can enclose a Cl− ion.[11] This structure has 5-coordinate, square pyramidal vanadium units linked together.
Isomerism
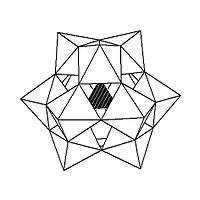
Isomerism is observed in some POMs. For example, the Keggin structure has 5 isomers, which are obtained by (conceptually) rotating one or more of the four M3O13 units through 60°.
| α-XM12O40n− | β-XM12O40n− | γ-XM12O40n− | δ-XM12O40n− | ε-XM12O40n− |
|---|---|---|---|---|
 |
 |
 |
 |
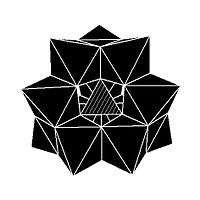 |
The five isomers of Keggin structure.
Lacunary structures
The structure of some POMs are derived from a larger POM's structure by removing one or more addenda atoms and their attendant oxide ions, giving a defective structure called a lacunary structure. An example of a compound with a Dawson lacunary structure is As2W15O56.[12] In 2014, vanadate species with similar, selective metal-binding properties have been reported.[13]
Polyoxotantalates, niobates, and vanadates
The properties of polyniobates and polytantatalates are similar, but substantially different from the polyoxovanadates. In fact, polyvanadates are more similar to the oxomolybdates and tungstates.[14]

The similarities between the polyniobates and polytantatalates arise primarily from the equivalence in the charge of their stable 5+ ion and their size (64 pm) due to the lanthanide contraction. The most common members are [M6O19]8- (M = Nb, Ta), which adopt the Lindqvist structure. These octaanions form in strongly basic conditions from alkali melts of the extended metal oxides (M2O5), or in the case of Nb even from mixtures of niobic acid and alkali metal hydroxides in aqueous solution. The hexatantalate can also be prepared by condensation of peroxotantalate [Ta(O2)4]3- in alkaline media.[15] These polyoxometalates display the an anomalous aqueous solubility trend of their alkali metal salts inasmuch as their Cs+ and Rb+ salts are more soluble than their Na+ and Li+ salts. The opposite trend is observed in group VI POMs.[16] The decametalates with the formula [M10O28]6- (M = Nb,[17] Ta[18]) are isostructural with decavanadate and are formed exclusively by edge-sharing {MO6} octahedra whereas the structure of decatungstate [W10O32]4- comprises edge-sharing and corner-sharing tungstate octahedra.
Oxoalkoxometalates
Polyoxometalates with addenda atoms outside Group 5 and 6 transition metals are known. Examples include the dodecatitanates Ti12O16(OPri)16 (where OPri stands for an alkoxy group),[19] the iron oxoalkoxometalates[20] and iron keggin ion.[21] These structures are also categorised as POMs,[9] and are known as polyoxoalkoxometalates due to the presence of the alkoxy groups.
Applications
POMs are employed as commercial catalysts for oxidation of organic compounds.[22][23]
Potential and emerging applications
The wide range of size, structure and elemental composition of polyoxometalates leads to a wide range of different properties and a corresponding wide range of potential applications
The Keggin ions are well-known to be thermally stable and reversibly reduced by accepting electrons. This makes them useful as catalysts for a range of organic reactions.
Like other polyanions, POMs engage weak- or non-bonding interactions.[24][25]
Spherical nanoporous polyoxomolybdate-based capsules of different types containing more than 100 metal atoms reported have distinctive properties regarding their assembly into vesicles and the chemistry which can be conducting within the pores and cavities.[1] A discrete polyoxometalate Lindqvist ion of the form W6O192− was imaged recently for the first time within the capillary of a carbon nanotube following steric locking of the anion with the tubule. In situ relaxation of the anion in its equatorial plane was demonstrated.[26]
Some POMs exhibit unusual magnetic properties[27] and are being investigated as possible nanocomputer storage devices (see qubits).[28]
Some potential "green" applications have been reported, such as a non-chlorine based, wood pulp bleaching process,[29] a method of decontaminating water.[30] and a method to catalytically produce formic acid from biomass (OxFA process).[31] Polyoxometalates have been shown to catalyse water splitting.[32]
Polyoxometalates can be reduced from conduction band of wide-band-gap semiconductors, which suggests that polyoxometalates may be useful in energy storage and conversions.[33]
Potential medicinal applications include anti-tumoral and anti-viral drugs.[34]
Experiments show that polyoxometalates can be "harvested" in the laboratory to construct nano-sized non-volatile (permanent) storage devices, also known as flash memory devices.[35][36]
References
- 1 2 3 From Scheele and Berzelius to Müller: polyoxometalates (POMs) revisited and the "missing link" between the bottom up and top down approaches P. Gouzerh, M. Che; L’Actualité Chimique, 2006, 298, 9
- ↑ The Structure and Formula of 12-Phosphotungstic Acid J.F. Keggin. Proc. Roy. Soc., A, 144, 851, 75-100 (1934) doi:10.1098/rspa.1934.0035
- ↑ Y.-F. Song, D.-L. Long, and L. Cronin, Non covalently connected frameworks with nanoscale channels assembled from a tethered polyoxometalate- pyrene hybrid, Angew. Chem. Int. Ed., 2007, 46, 3900-3904 doi:10.1002/anie.200604734.
- ↑ A novel 3D organic–inorganic hybrid based on sandwich-type cadmium hetereopolymolybdate: [Cd4(H2O)2(2,2′-bpy)2] Cd[Mo6O12(OH)3(PO4)2(HPO4)2]2 [Mo2O4(2,2′-bpy)2]2·3H2O, Hong-Xu Guo and Shi-Xiong Liu Inorganic Chemistry Communications 7, 11, (2004), 1217 doi:10.1016/j.inoche.2004.09.010
- ↑ Classical and Quantum Magnetism in Giant Keplerate Magnetic Molecules Achim Müller, Marshall Luban, Robert Modler, Paul Kögerler, Maria Axenovich, Jürgen Schnack, Paul Canfield, Sergey Bud'ko, Neil Harrison. ChemPhysChem 2001, 2, 517.
- ↑ Field-dependent magnetic parameters in Ni4Mo12: Magnetostriction at the molecular level? Jürgen Schnack, Mirko Brüger, Marshall Luban, Paul Kögerler, Emilia Morosan, Ronald Fuchs, Robert Modler, Hiroyuki Nojiri, Ram C. Rai, Jinbo Cao, Janice L. Musfeldt, Xing Wei. Phys. Rev. B 2006, 73, 094401.
- ↑ Direct Bromination of Keggin Fragments To Give [PW9O28Br6]3−: A Polyoxotungstate with a Hexabrominated Face R. John Errington, Richard L. Wingad, William Clegg, Mark R. J. Elsegood Angewandte Chemie 39, volume 21 ,3884 – 3886 doi:10.1002/1521-3773(20001103)39:21<3884::AID-ANIE3884>3.0.CO;2-M
- ↑ Functionalization of polyoxomolybdates: the example of nitrosyl derivatives P. Gouzerh, Y. Jeannin, A. Proust, F. Robert and S. G. Roh Molecular Engineering 3, (1993) 79 doi:10.1007/BF00999625
- 1 2 Polyoxometalates: From Platonic Solids to Anti-Retroviral Activity by Michael Thor Pope, Achim Müller, Springer (1994) ISBN 0-7923-2421-8
- ↑ A New Structural Type for Heteropoly Anions. The Crystal Structure of (NH4)2H6(CeMo12O42)12H2O DD Dexter, JV Silverton - Journal of the American Chemical Society, 1968, 3589
- ↑ Supramolecular Inorganic Chemistry: Small Guests in Small and Large Hosts A. Müller, H. Reuter, S. Dillinger. Angew. Chem. Int. Ed. Engl. 1995, 34, 2328.
- ↑ Manganous heteropolytungstates. Synthesis and heteroatom effects in Wells–Dawson-derived sandwich complexes I.M. Mbomekalle, B. Keita, L. Nadjo, P. Berthet, W. A. Neiwert, C.L. Hill, M.D. Ritorto and T. M. Anderson, Dalton Trans., 2003, 2646 - 2650, doi:10.1039/b304255c
- ↑ A Molecular Placeholder Strategy To Access a Family of Transition-Metal-Functionalized Vanadium Oxide Clusters, K. Kastner, J. T. Margraf, T. Clark, C. Streb, Chem. Eur. J., 2014, 20, 12269-12273 doi: 10.1002/chem.201403592
- ↑ "Hetero and lacunary polyoxovanadate chemistry: Synthesis, reactivity and structural aspects" Coordination Chemistry Reviews 2011, Volume 255, Pages 2270–2280. doi: 10.1016/j.ccr.2011.02.013
- ↑ L.B Fullmer, P.I. Molina, M.R. Antonio, M. Nyman, Dalton Transactions, 2014, doi: 10.1039/C4DT02394C
- ↑ T. M. Anderson, S. G. Thoma, F. Bonhomme, M. A. Rodriguez, H. Park, J. B. Parise, T. M. Alan, J. P. Larentzos, M. Nyman, Crystal Growth & Design, 2007, doi: 10.1021/cg0606904
- ↑ E. J. Graeber, B. Morosin, Acta Crystallographyca B, 1977, doi: 10.1107/S0567740877007900
- ↑ M. Matsumoto, Y. Ozawa, A. Yagasaki, Y. Zhe, Inorganic Chemistry, 2013, doi: 10.1021/ic400864e
- ↑ Dodecatitanates: a new family of stable polyoxotitanates V. W. Day, T. A. Eberspacher, W. G. Klemperer, and C. W. Park J. Am. Chem. Soc.; 1993; 115(18) pp 8469 - 8470; doi:10.1021/ja00071a075
- ↑ Synthesis and Structure of [Fe13O4F24(OMe)12]5−: The First Open-Shell Keggin Ion Avi Bino, Michael Ardon, Dongwhan Lee, Bernhard Spingler, and Stephen J. Lippard J. Am. Chem. Soc., 124 (17), 4578 -4579, 2002doi:10.1021/ja025590a
- ↑ Aqueous formation and manipulation of the iron-oxo Keggin ion Omid Sadeghi, Lev N. Zakharov and May Nyman, Science; 2015; 347 (6228) pp 1359 - 1362; doi:10.1126/science.aaa4620
- ↑ Makoto Misono "Catalytic chemistry of solid polyoxometalates and their industrial applications" Molecular Engineering 1993, Volume 3, pp 193-203. doi:10.1007/BF00999633
- ↑ Ivan V. Kozhevnikov "Catalysis by Heteropoly Acids and Multicomponent Polyoxometalates in Liquid-Phase Reactions" Chem. Rev., 1998, volume 98, pp 171–198. doi:10.1021/cr960400y
- ↑ Role of H-Bonded Interactions in the Crystal Packing of Phenylenediammonium Phosphomolybdates Shailesh Upreti and Arunachalam Ramanan, Crystal Growth & Design, 2006, 6(9), 2066-2071 doi:10.1021/cg0601610
- ↑ Structure-Directing Role of Hydrogen-Bonded Dimers of Phenylenediammonium Cations: Supramolecular Assemblies of Octamolybdate-Based Organic-Inorganic Hybrids, Shailesh Upreti and Arunachalam Ramanan, Crystal Growth & Design, 2005, 5(5), 1837–1843 doi:10.1021/cg050100m
- ↑ Direct Imaging of the Structure, Relaxation, and Sterically Constrained Motion of Encapsulated Tungsten Polyoxometalate Lindqvist Ions within Carbon Nanotubes, Jeremy Sloan, Gemma Matthewman, Clare Dyer-Smith, A-Young Sung, Zheng Liu, Kazu Suenaga, Angus I. Kirkland, and Emmanuel Flahaut, ACS Nano, 2(5), 966–976, 2008. doi:10.1021/nn7002508
- ↑ Polyoxovanadates: High-Nuclearity Spin Clusters with Interesting Host-Guest Systems and Different Electron Populations. Synthesis, Spin Organization, Magnetochemistry, and Spectroscopic Studies A Müller, R Sessoli,E Krickemeyer, H Bögge, J Meyer, D Gatteschi, L Pardi, J Westphal, K Hovemeier, R Rohlfing, J Döring, F Hellweg, C Beugholt and M Schmidtmann Inorg. Chem., 36 (23), 5239 -5250, 1997.
- ↑ Spin qubits with electrically gated polyoxometalate molecule J. Lehmann, A. Gaita-Ariño, E. Coronado, D. Loss Nature Nanotechnology 2, 312 - 317 (2007) doi:10.1038/nnano.2007.110
- ↑ Alternatives for lignocellulosic pulp delignification using polyoxometalates and oxygen: a review A. R. Gaspar, J. A. F. Gamelas, D. V. Evtuguin and C P Neto Green Chem., 2007, 9, 717 - 730, doi:10.1039/b607824a
- ↑ Polyoxometallate photocatalysis for decontaminating the aquatic environment from organic and inorganic pollutants A. Hiskia, A.Troupis, S. Antonaraki, E. Gkika, P. Kormali, E. Papaconstantinou, International Journal of Environmental Analytical Chemistry, 86, Issue 3 & 4 (2006), 233, doi:10.1080/03067310500247520
- ↑ R. Wölfel, N. Taccardi, A. Bösmann, P. Wasserscheid (2011). "Selective catalytic conversion of biobased carbohydrates to formic acid using molecular oxygen". Green Chem. (13): 2759. doi:10.1039/C1GC15434F.
- ↑ Decoupled catalytic hydrogen evolution from a molecular metal oxide redox mediator in water splitting B. Rausch, M. D. Symes, G. Chisholm, L. Cronin, Science, 345, 1326-1330, 2014.
- ↑ Investigation of the photocatalytic activity of TiO2–polyoxometalate systems for the oxidation of methanol Chaokang Gu, Curtis Shannon Journal of Molecular Catalysis A: Chemical, 262 (1-2), 185–189, 2007. doi:10.1016/j.molcata.2006.08.029
- ↑ Polyoxometalates in Medicine Jeffrey T. Rhule, Craig L. Hill, and Deborah A. Judd Chem. Rev., 98 (1), 327 -358, 1998.
- ↑ Flash memory breaches nanoscales http://www.thehindu.com/sci-tech/technology/flash-memory-breaches-nanoscales/article6615239.ece
- ↑ Design and fabrication of memory devices based on nanoscale polyoxometalate clusters C. Busche, L. Vila-Nadal, J. Yan, H. N. Miras, D.-L. Long, V. P. Georgiev, A. Asenov, R. H. Pedersen, N. Gadegaard, M. M. Mirza, D. J. Paul, J. M. Poblet, L. Cronin, Nature, 515 , 545-549, 2014.
Further reading
- D. L. Long, E. Burkholder, and L. Cronin, 'Polyoxometalate clusters, nanostructures and materials: From self-assembly to designer materials and devices', Chem. Soc. Rev., 2007, 36, 105-121. doi:10.1039/b502666k
- M.T. Pope "Heteropoly and Isopoly Oxometalates", Springer Verlag, New York, (1983).
- M.T. Pope, A. Müller, Polyoxometalate Chemistry: An Old Field with New Dimensions in Several Disciplines, Angew. Chem. Int. Ed. Engl. 1991, 30, 34.
- Special volume on "Polyoxometalates", Chem.Rev.,1998, 98, 1
- Special POM issue: L. Cronin, A. Müller (guest eds.), Chem. Soc. Rev., 2012, 7325-7648