Plastic

Generic term used in the case of polymeric material that may contain other substances
to improve performance and/or reduce costs.
Note 1: The use of this term instead of polymer is a source of confusion and thus is
not recommended.
Note 2: This term is used in polymer engineering for materials often compounded that
can be processed by flow.[1]
Plastic is a material consisting of any of a wide range of synthetic or semi-synthetic organics that are malleable and can be molded into solid objects of diverse shapes. Plastics are typically organic polymers of high molecular mass, but they often contain other substances. They are usually synthetic, most commonly derived from petrochemicals, but many are partially natural.[2] Plasticity is the general property of all materials that are able to irreversibly deform without breaking, but this occurs to such a degree with this class of moldable polymers that their name is an emphasis on this ability.
Due to their relatively low cost, ease of manufacture, versatility, and imperviousness to water, plastics are used in an enormous and expanding range of products, from paper clips to spaceships. They have already displaced many traditional materials, such as wood, stone, horn and bone, leather, paper, metal, glass, and ceramic, in most of their former uses. In developed countries, about a third of plastic is used in packaging and another third in buildings such as piping used in plumbing or vinyl siding.[3] Other uses include automobiles (up to 20% plastic[3]), furniture, and toys.[3] In the developing world, the ratios may be different - for example, reportedly 42% of India's consumption is used in packaging.[3] Plastics have many uses in the medical field as well, to include polymer implants, however the field of plastic surgery is not named for use of plastic material, but rather the more generic meaning of the word plasticity in regards to the reshaping of flesh.
The world's first fully synthetic plastic was bakelite, invented in New York in 1907 by Leo Baekeland[4] who coined the term 'plastics'.[5] Many chemists contributed to the materials science of plastics, including Nobel laureate Hermann Staudinger who has been called "the father of polymer chemistry" and Herman Mark, known as "the father of polymer physics".[6] The success and dominance of plastics starting in the early 20th century led to environmental concerns regarding its slow decomposition rate after being discarded as trash due to its composition of very large molecules. Toward the end of the century, one approach to this problem was met with wide efforts toward recycling.
Etymology
The word plastic is derived from the Greek πλαστικός (plastikos) meaning "capable of being shaped or molded", from πλαστός (plastos) meaning "molded".[7][8] It refers to their malleability, or plasticity during manufacture, that allows them to be cast, pressed, or extruded into a variety of shapes—such as films, fibers, plates, tubes, bottles, boxes, and much more.
The common word plastic should not be confused with the technical adjective plastic, which is applied to any material which undergoes a permanent change of shape (plastic deformation) when strained beyond a certain point. Aluminum which is stamped or forged, for instance, exhibits plasticity in this sense, but is not plastic in the common sense; in contrast, in their finished forms, some plastics will break before deforming and therefore are not plastic in the technical sense.
Common plastics and uses


- Polyester (PES) – Fibers, textiles.
- Polyethylene terephthalate (PET) – Carbonated drinks bottles, peanut butter jars, plastic film, microwavable packaging.
- Polyethylene (PE) – Wide range of inexpensive uses including supermarket bags, plastic bottles.
- High-density polyethylene (HDPE) – Detergent bottles, milk jugs, and molded plastic cases.
- Polyvinyl chloride (PVC) – Plumbing pipes and guttering, shower curtains, window frames, flooring.
- Polyvinylidene chloride (PVDC) (Saran) – Food packaging.
- Low-density polyethylene (LDPE) – Outdoor furniture, siding, floor tiles, shower curtains, clamshell packaging.
- Polypropylene (PP) – Bottle caps, drinking straws, yogurt containers, appliances, car fenders (bumpers), plastic pressure pipe systems.
- Polystyrene (PS) – Packaging foam/"peanuts", food containers, plastic tableware, disposable cups, plates, cutlery, CD and cassette boxes.
- High impact polystyrene (HIPS) -: Refrigerator liners, food packaging, vending cups.
- Polyamides (PA) (Nylons) – Fibers, toothbrush bristles, tubing, fishing line, low strength machine parts: under-the-hood car engine parts or gun frames.
- Acrylonitrile butadiene styrene (ABS) – Electronic equipment cases (e.g., computer monitors, printers, keyboards), drainage pipe.
- Polyethylene/Acrylonitrile Butadiene Styrene (PE/ABS) – A slippery blend of PE and ABS used in low-duty dry bearings.
- Polycarbonate (PC) – Compact discs, eyeglasses, riot shields, security windows, traffic lights, lenses.
- Polycarbonate/Acrylonitrile Butadiene Styrene (PC/ABS) – A blend of PC and ABS that creates a stronger plastic. Used in car interior and exterior parts, and mobile phone bodies.
- Polyurethanes (PU) – Cushioning foams, thermal insulation foams, surface coatings, printing rollers (Currently 6th or 7th most commonly used plastic material, for instance the most commonly used plastic in cars).
Special purpose plastics
- Maleimide/Bismaleimide Used in high temperature composite materials.
- Melamine formaldehyde (MF) – One of the aminoplasts, and used as a multi-colorable alternative to phenolics, for instance in moldings (e.g., break-resistance alternatives to ceramic cups, plates and bowls for children) and the decorated top surface layer of the paper laminates (e.g., Formica).
- Plastarch material – Biodegradable and heat resistant, thermoplastic composed of modified corn starch.
- Phenolics (PF) or (phenol formaldehydes) – High modulus, relatively heat resistant, and excellent fire resistant polymer. Used for insulating parts in electrical fixtures, paper laminated products (e.g., Formica), thermally insulation foams. It is a thermosetting plastic, with the familiar trade name Bakelite, that can be molded by heat and pressure when mixed with a filler-like wood flour or can be cast in its unfilled liquid form or cast as foam (e.g., Oasis). Problems include the probability of moldings naturally being dark colors (red, green, brown), and as thermoset it is difficult to recycle.
- Polyepoxide (Epoxy) Used as an adhesive, potting agent for electrical components, and matrix for composite materials with hardeners including amine, amide, and Boron Trifluoride.
- Polyetheretherketone (PEEK) – Strong, chemical- and heat-resistant thermoplastic, biocompatibility allows for use in medical implant applications, aerospace moldings. One of the most expensive commercial polymers.
- Polyetherimide (PEI) (Ultem) – A high temperature, chemically stable polymer that does not crystallize.
- Polyimide—A High temperature plastic used in materials such as Kapton tape.
- Polylactic acid (PLA) – A biodegradable, thermoplastic found converted into a variety of aliphatic polyesters derived from lactic acid which in turn can be made by fermentation of various agricultural products such as corn starch, once made from dairy products.
- Polymethyl methacrylate (PMMA) (Acrylic) – Contact lenses (of the original "hard" variety), glazing (best known in this form by its various trade names around the world; e.g., Perspex, Oroglas, Plexiglas), aglets, fluorescent light diffusers, rear light covers for vehicles. It forms the basis of artistic and commercial acrylic paints when suspended in water with the use of other agents.
- Polytetrafluoroethylene (PTFE) – Heat-resistant, low-friction coatings, used in things like non-stick surfaces for frying pans, plumber's tape and water slides. It is more commonly known as Teflon.
- Urea-formaldehyde (UF) – One of the aminoplasts and used as a multi-colorable alternative to phenolics. Used as a wood adhesive (for plywood, chipboard, hardboard) and electrical switch housings.
- Furan—Resin based on Furfuryl Alcohol used in foundry sands and biologically derived composites.
- Silicone—Heat resistant resin used mainly as a sealant but also used for high temperature cooking utensils and as a base resin for industrial paints.
- Polysulfone—High temperature melt processable resin used in membranes, filtration media, water heater dip tubes and other high temperature applications.
History
_bowl%2C_by_GEECO%2C_Made_in_England%2C_c1950.jpg)
The development of plastics has evolved from the use of natural plastic materials (e.g., chewing gum, shellac) to the use of chemically modified, natural materials (e.g., natural rubber, nitrocellulose, collagen, galalite) and finally to completely synthetic molecules (e.g., bakelite, epoxy, Polyvinyl chloride). Early plastics were bio-derived materials such as egg and blood proteins, which are organic polymers. In 1600 BC, Mesoamericans used natural rubber for balls, bands, and figurines.[3] Treated cattle horns were used as windows for lanterns in the Middle Ages. Materials that mimicked the properties of horns were developed by treating milk-proteins (casein) with lye.
In the 1800s, as industrial chemistry developed during the Industrial Revolution, many materials were reported. The development of plastics also accelerated with Charles Goodyear's discovery of vulcanization to thermoset materials derived from natural rubber.
Parkesine is considered the first man-made plastic. The plastic material was patented by Alexander Parkes, In Birmingham, UK in 1856.[9] It was unveiled at the 1862 Great International Exhibition in London.[10] Parkesine won a bronze medal at the 1862 World's fair in London. Parkesine was made from cellulose (the major component of plant cell walls) treated with nitric acid as a solvent. The output of the process (commonly known as cellulose nitrate or pyroxilin) could be dissolved in alcohol and hardened into a transparent and elastic material that could be molded when heated.[11] By incorporating pigments into the product, it could be made to resemble ivory.
In 1897, the Hanover, Germany mass printing press owner Wilhelm Krische was commissioned to develop an alternative to blackboards.[12] The resultant horn-like plastic made from the milk protein casein was developed in cooperation with the Austrian chemist (Friedrich) Adolph Spitteler (1846–1940). The final result was unsuitable for the original purpose.[13] In 1893, French chemist Auguste Trillat discovered the means to insolubilize casein by immersion in formaldehyde, producing material marketed as galalith.[12]
In the early 1900s, Bakelite, the first fully synthetic thermoset, was reported by Belgian chemist Leo Baekeland by using phenol and formaldehyde.
After World War I, improvements in chemical technology led to an explosion in new forms of plastics, with mass production beginning in the 1940s and 1950s (around World War II).[14] Among the earliest examples in the wave of new polymers were polystyrene (PS), first produced by BASF in the 1930s,[3] and polyvinyl chloride (PVC), first created in 1872 but commercially produced in the late 1920s.[3] In 1923, Durite Plastics Inc. was the first manufacturer of phenol-furfural resins.[15] In 1933, polyethylene was discovered by Imperial Chemical Industries (ICI) researchers Reginald Gibson and Eric Fawcett.[3]
In 1954, Polypropylene was discovered by Giulio Natta and began to be manufactured in 1957.[3]
In 1954, expanded polystyrene (used for building insulation, packaging, and cups) was invented by Dow Chemical.[3]
Polyethylene terephthalate (PET)'s discovery is credited to employees of the Calico Printers' Association in the UK in 1941; it was licensed to DuPont for the USA and ICI otherwise, and as one of the few plastics appropriate as a replacement for glass in many circumstances, resulting in widespread use for bottles in Europe.[3]
Composition
Most plastics contain organic polymers. The vast majority of these polymers are based on chains of carbon atoms alone or with oxygen, sulfur, or nitrogen as well. The backbone is that part of the chain on the main "path" linking a large number of repeat units together. To customize the properties of a plastic, different molecular groups "hang" from the backbone (usually they are "hung" as part of the monomers before the monomers are linked together to form the polymer chain). The structure of these "side chains" influence the properties of the polymer. This fine tuning of the repeating unit's molecular structure influences the properties of the polymer.
Most plastics contain other organic or inorganic compounds blended in. The amount of additives ranges from zero percentage (for example in polymers used to wrap foods) to more than 50% for certain electronic applications. The average content of additives is 20% by weight of the polymer.
Many of the controversies associated with plastics are associated with the additives.[16] Organotin compounds are particularly toxic.[17]
Fillers
Fillers improve performance and/or reduce production costs. Stabilizing additives include fire retardants to lower the flammability of the material. Many plastics contain fillers, relatively inert and inexpensive materials that make the product cheaper by weight.
Typically fillers are mineral in origin, e.g., chalk. Some fillers are more chemically active and are called reinforcing agents. Other fillers include zinc oxide, wood flour, ivory dust, cellulose and starch.[18]
Plasticizers
Since many organic polymers are too rigid for particular applications, they are blended with plasticizers (the largest group of additives[17]), oily compounds that confer improved rheology.
Colorants
Colorants are common additives, although their weight contribution is small.
Classification
Plastics are usually classified by their chemical structure of the polymer's backbone and side chains. Some important groups in these classifications are the acrylics, polyesters, silicones, polyurethanes, and halogenated plastics. Plastics can also be classified by the chemical process used in their synthesis, such as condensation, polyaddition, and cross-linking.[19]
Thermoplastics and thermosetting polymers
There are two types of plastics: thermoplastics and thermosetting polymers. Thermoplastics are the plastics that do not undergo chemical change in their composition when heated and can be molded again and again. Examples include polyethylene, polypropylene, polystyrene and polyvinyl chloride.[20] Common thermoplastics range from 20,000 to 500,000 amu, while thermosets are assumed to have infinite molecular weight. These chains are made up of many repeating molecular units, known as repeat units, derived from monomers; each polymer chain will have several thousand repeating units.
Thermosets can melt and take shape once; after they have solidified, they stay solid. In the thermosetting process, a chemical reaction occurs that is irreversible. The vulcanization of rubber is a thermosetting process. Before heating with sulfur, the polyisoprene is a tacky, slightly runny material, but after vulcanization the product is rigid and non-tacky.
Other classifications
Other classifications are based on qualities that are relevant for manufacturing or product design. Examples of such classes are the thermoplastic and thermoset, elastomer, structural, biodegradable, and electrically conductive. Plastics can also be classified by various physical properties, such as density, tensile strength, glass transition temperature, and resistance to various chemical products.
Biodegradability
Biodegradable plastics break down (degrade) upon exposure to sunlight (e.g., ultra-violet radiation), water or dampness, bacteria, enzymes, wind abrasion, and in some instances, rodent, pest, or insect attack are also included as forms of biodegradation or environmental degradation. Some modes of degradation require that the plastic be exposed at the surface, whereas other modes will only be effective if certain conditions exist in landfill or composting systems. Starch powder has been mixed with plastic as a filler to allow it to degrade more easily, but it still does not lead to complete breakdown of the plastic. Some researchers have actually genetically engineered bacteria that synthesize a completely biodegradable plastic, but this material, such as Biopol, is expensive at present.[21] Companies have made biodegradable additives to enhance the biodegradation of plastics.
Natural vs synthetic
Most plastics are produced from petrochemicals. Motivated by the finiteness of petrochemical reserves and threat of global warming, bioplastics are being developed. Bioplastics are made substantially from renewable plant materials such as cellulose and starch.[22]
In comparison to the global consumption of all flexible packaging, estimated at 12.3 million tonnes/year, estimates put global production capacity at 327,000 tonnes/year for related bio-derived materials.[23][24]
Crystalline vs amorphous
Some plastics are partially crystalline and partially amorphous in molecular structure, giving them both a melting point (the temperature at which the attractive intermolecular forces are overcome) and one or more glass transitions (temperatures above which the extent of localized molecular flexibility is substantially increased). The so-called semi-crystalline plastics include polyethylene, polypropylene, poly (vinyl chloride), polyamides (nylons), polyesters and some polyurethanes. Many plastics are completely amorphous, such as polystyrene and its copolymers, poly (methyl methacrylate), and all thermosets.
Representative polymers


Bakelite
The first plastic based on a synthetic polymer was made from phenol and formaldehyde, with the first viable and cheap synthesis methods invented in 1907, by Leo Hendrik Baekeland, a Belgian-born American living in New York state. Baekeland was looking for an insulating shellac to coat wires in electric motors and generators. He found that combining phenol (C6H5OH) and formaldehyde (HCOH) formed a sticky mass and later found that the material could be mixed with wood flour, asbestos, or slate dust to create strong and fire resistant "composite" materials. The new material tended to foam during synthesis, requiring that Baekeland build pressure vessels to force out the bubbles and provide a smooth, uniform product, as he announced in 1909, in a meeting of the American Chemical Society.[25] Bakelite was originally used for electrical and mechanical parts, coming into widespread use in consumer goods and jewelry in the 1920s. Bakelite was a purely synthetic material, not derived from living matter. It was also an early thermosetting plastic.
Polystyrene
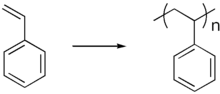
Unplasticised polystyrene is a rigid, brittle, inexpensive plastic that has been used to make plastic model kits and similar knick-knacks. It also is the basis for some of the most popular "foamed" plastics, under the name styrene foam or Styrofoam. Like most other foam plastics, foamed polystyrene can be manufactured in an "open cell" form, in which the foam bubbles are interconnected, as in an absorbent sponge, and "closed cell", in which all the bubbles are distinct, like tiny balloons, as in gas-filled foam insulation and flotation devices. In the late 1950s, high impact styrene was introduced, which was not brittle. It finds much current use as the substance of toy figurines and novelties.
Polyvinyl chloride

Polyvinyl chloride (PVC, commonly called "vinyl")[26] incorporates chlorine atoms. The C-Cl bonds in the backbone are hydrophobic and resist oxidation (and burning). PVC is stiff, strong, heat and weather resistant, properties that recommend its use in devices for plumbing, gutters, house siding, enclosures for computers and other electronics gear. PVC can also be softened with chemical processing, and in this form it is now used for shrink-wrap, food packaging, and rain gear.
All PVC polymers are degraded by heat and light. When this happens, hydrogen chloride is released into the atmosphere and oxidation of the compound occurs.[27] Because hydrogen chloride readily combines with water vapor in the air to form hydrochloric acid,[28] polyvinyl chloride is not recommended for long-term archival storage of silver, photographic film or paper (mylar is preferable).[29]
Nylon
The plastics industry was revolutionized in the 1930s with the announcement of polyamide (PA), far better known by its trade name nylon. Nylon was the first purely synthetic fiber, introduced by DuPont Corporation at the 1939 World's Fair in New York City.
In 1927, DuPont had begun a secret development project designated Fiber66, under the direction of Harvard chemist Wallace Carothers and chemistry department director Elmer Keiser Bolton. Carothers had been hired to perform pure research, and he worked to understand the new materials' molecular structure and physical properties. He took some of the first steps in the molecular design of the materials.
His work led to the discovery of synthetic nylon fiber, which was very strong but also very flexible. The first application was for bristles for toothbrushes. However, Du Pont's real target was silk, particularly silk stockings. Carothers and his team synthesized a number of different polyamides including polyamide 6.6 and 4.6, as well as polyesters.[30]

It took DuPont twelve years and US$27 million to refine nylon, and to synthesize and develop the industrial processes for bulk manufacture. With such a major investment, it was no surprise that Du Pont spared little expense to promote nylon after its introduction, creating a public sensation, or "nylon mania".
Nylon mania came to an abrupt stop at the end of 1941 when the USA entered World War II. The production capacity that had been built up to produce nylon stockings, or just nylons, for American women was taken over to manufacture vast numbers of parachutes for fliers and paratroopers. After the war ended, DuPont went back to selling nylon to the public, engaging in another promotional campaign in 1946 that resulted in an even bigger craze, triggering the so-called nylon riots.
Subsequently polyamides 6, 10, 11, and 12 have been developed based on monomers which are ring compounds; e.g. caprolactam. Nylon 66 is a material manufactured by condensation polymerization.
Nylons still remain important plastics, and not just for use in fabrics. In its bulk form it is very wear resistant, particularly if oil-impregnated, and so is used to build gears, plain bearings, valve seats, seals and because of good heat-resistance, increasingly for under-the-hood applications in cars, and other mechanical parts.
Poly(methyl methacrylate)
Poly(methyl methacrylate) (PMMA), also known as acrylic or acrylic glass as well as by the trade names Plexiglas, Acrylite, Lucite, and Perspex among several others (see below), is a transparent thermoplastic often used in sheet form as a lightweight or shatter-resistant alternative to glass. The same material can be utilised as a casting resin, in inks and coatings, and has many other uses.
Rubber
Natural rubber is an elastomer (an elastic hydrocarbon polymer) that originally was derived from latex, a milky colloidal suspension found in specialised vessels in some plants. It is useful directly in this form (indeed, the first appearance of rubber in Europe was cloth waterproofed with unvulcanized latex from Brazil). However, in 1839, Charles Goodyear invented vulcanized rubber; a form of natural rubber heated with sulfur (and a few other chemicals), forming cross-links between polymer chains (vulcanization), improving elasticity and durability.
In 1851, Nelson Goodyear added fillers to natural rubber materials to form ebonite.[18]
Synthetic rubber
The first fully synthetic rubber was synthesized by Sergei Lebedev in 1910. In World War II, supply blockades of natural rubber from South East Asia caused a boom in development of synthetic rubber, notably styrene-butadiene rubber. In 1941, annual production of synthetic rubber in the U.S. was only 231 tonnes which increased to 840,000 tonnes in 1945. In the space race and nuclear arms race, Caltech researchers experimented with using synthetic rubbers for solid fuel for rockets. Ultimately, all large military rockets and missiles would use synthetic rubber based solid fuels, and they would also play a significant part in the civilian space effort.
Properties of plastics
The properties of plastics are defined chiefly by the organic chemistry of the polymer such as hardness, density, and resistance to heat, organic solvents, oxidation, and ionizing radiation. In particular, most plastics will melt upon heating to a few hundred degrees celsius.[31] While plastics can be made electrically conductive, with the conductivity of up to 80 kS/cm in stretch-oriented polyacetylene,[32] they are still no match for most metals like copper which have conductivities of several hundreds kS/cm.
UL Standards
Many properties of plastics are determined by tests as specified by Underwriters Laboratories, such as:
- Flammability - UL94
- High voltage arc tracking rate - UL746A
- Comparative Tracking Index
ISO
Many properties of plastics are determined by standards as specified by ISO, such as:
- ISO 306 - Thermoplastics
Toxicity
Pure plastics have low toxicity due to their insolubility in water and because they are biochemically inert, due to a large molecular weight. Plastic products contain a variety of additives, some of which can be toxic. For example, plasticizers like adipates and phthalates are often added to brittle plastics like polyvinyl chloride to make them pliable enough for use in food packaging, toys, and many other items. Traces of these compounds can leach out of the product. Owing to concerns over the effects of such leachates, the European Union has restricted the use of DEHP (di-2-ethylhexyl phthalate) and other phthalates in some applications, and the United States has limited the use of DEHP, DPB, BBP, DINP, DIDP, and DnOP in children's toys and child care articles with the Consumer Product Safety Improvement Act. Some compounds leaching from polystyrene food containers have been proposed to interfere with hormone functions and are suspected human carcinogens.[33] Other chemicals of potential concern include alkylphenols.[17]
Whereas the finished plastic may be non-toxic, the monomers used in the manufacture of the parent polymers may be toxic. In some cases, small amounts of those chemicals can remain trapped in the product unless suitable processing is employed. For example, the World Health Organization's International Agency for Research on Cancer (IARC) has recognized vinyl chloride, the precursor to PVC, as a human carcinogen.[33]
Bisphenol A
Some polymers may also decompose into the monomers or other toxic substances when heated. In 2011, it was reported that "almost all plastic products" sampled released chemicals with estrogenic activity, although the researchers identified plastics which did not leach chemicals with estrogenic activity.[34]
The primary building block of polycarbonates, bisphenol A (BPA), is an estrogen-like endocrine disruptor that may leach into food.[33] Research in Environmental Health Perspectives finds that BPA leached from the lining of tin cans, dental sealants and polycarbonate bottles can increase body weight of lab animals' offspring.[35] A more recent animal study suggests that even low-level exposure to BPA results in insulin resistance, which can lead to inflammation and heart disease.[36]
As of January 2010, the LA Times newspaper reports that the United States FDA is spending $30 million to investigate indications of BPA being linked to cancer.[37]
Bis(2-ethylhexyl) adipate, present in plastic wrap based on PVC, is also of concern, as are the volatile organic compounds present in new car smell.
The European Union has a permanent ban on the use of phthalates in toys. In 2009, the United States government banned certain types of phthalates commonly used in plastic.[38]
Environmental effects
Most plastics are durable and degrade very slowly; the very chemical bonds that make them so durable tend to make them resistant to most natural processes of degradation. However, microbial species and communities capable of degrading plastics are discovered from time to time, and some show promise as being useful for bioremediating certain classes of plastic waste.
- In 1975 a team of Japanese scientists studying ponds containing waste water from a nylon factory, discovered a strain of Flavobacterium that digested certain byproducts of nylon 6 manufacture, such as the linear dimer of 6-aminohexanoate.[39] Nylon 4 or polybutyrolactam can be degraded by the (ND-10 and ND-11) strands of Pseudomonas sp. found in sludge. This produced γ-aminobutyric acid (GABA) as a byproduct.[40]
- Several species of soil fungi can consume polyurethane.[41] This includes two species of the Ecuadorian fungus Pestalotiopsis that can consume polyurethane aerobically and also in anaerobic conditions such as those at the bottom of landfills.[42]
- Methanogenic consortia degrade styrene, using it as a carbon source.[43] Pseudomonas putida can convert styrene oil into various biodegradable polyhydroxyalkanoates.[44][45]
- Microbial communities isolated from soil samples mixed with starch have been shown to capable of degrading polypropylene.[46]
- The fungus Aspergillus fumigatus effectively degrades plasticized PVC.[47] Phanerochaete chrysosporium was grown on PVC in a mineral salt agar.[48] Phanerochaete chrysosporium, Lentinus tigrinus, Aspergillus niger, and Aspergillus sydowii can also effectively degrade PVC.[49] Phanerochaete chrysosporium was grown on PVC in a mineral salt agar.[48]
- Acinetobacter has been found to partially degrade low molecular weight polyethylene oligomers.[50] When used in combination, Pseudomonas fluorescens and Sphingomonas can degrade over 40% of the weight of plastic bags in less than three months.[51] The thermophilic bacterium Brevibacillus borstelensis (strain 707) was isolated from a soil sample and found capable of using low-density polyethylene as a sole carbon source when incubated at 50 degrees Celsius. Pre-exposure of the plastic to ultraviolet radiation broke chemical bonds and aided biodegradation; the longer the period of UV exposure, the greater the promotion of the degradation.[52]
- Less desirably, hazardous molds have been found aboard space stations, molds that degrade rubber into a digestible form.[53]
- Several species of yeasts, bacteria, algae and lichens have been found growing on synthetic polymer artifacts in museums and at archaeological sites.[54]
- In the plastic-polluted waters of the Sargasso Sea, bacteria have been found that consume various types of plastic; however it is unknown to what extent these bacteria effectively clean up poisons rather than simply releasing them into the marine microbial ecosystem.
- Plastic eating microbes also have been found in landfills.[55]
- Nocardia can degrade PET with an esterase enzyme.[56]
- The fungi Geotrichum candidum, found in Belize, has been found to consume the polycarbonate plastic found in CD's.[57][58]
- Phenol-formaldehyde, commonly known as bakelite, is degraded by the white rot fungus Phanerochaete chrysosporium [59]
- The futuro house was made of fibreglass-reinforced polyesters, polyester-polyurethane, and poly(methylmethacrylate.) One such house was found to be harmfully degraded by Cyanobacteria and Archaea.[60][61]
Since the 1950s, one billion tons of plastic have been discarded and some of that material might persist for centuries or much longer, as is demonstrated by the persistence of natural materials such as amber.[62]
Serious environmental threats from plastic have been suggested in the light of the increasing presence of microplastics in the marine food chain along with many highly toxic chemical pollutants that accumulate in plastics. They also accumulate in larger fragmented pieces of plastic called nurdles.[63] In the 1960s the latter were observed in the guts of seabirds, and since then have been found in increasing concentration.[64] In 2009, it was estimated that 10% of modern waste was plastics,[14] although estimates vary according to region.[64] Meanwhile, 50-80% of debris in marine areas is plastic.[64]
Before the ban on the use of CFCs in extrusion of polystyrene (and in general use, except in life-critical fire suppression systems; see Montreal Protocol), the production of polystyrene contributed to the depletion of the ozone layer, but current extrusion processes use non-CFCs.
Climate change
The effect of plastics on global warming is mixed. Plastics are generally made from petroleum. If the plastic is incinerated, it increases carbon emissions; if it is placed in a landfill, it becomes a carbon sink[65] although biodegradable plastics have caused methane emissions.[66] Due to the lightness of plastic versus glass or metal, plastic may reduce energy consumption. For example, packaging beverages in PET plastic rather than glass or metal is estimated to save 52% in transportation energy.[3]
Production of plastics
Production of plastics from crude oil requires 62 to 108 MJ of energy per kilogram (taking into account the average efficiency of US utility stations of 35%). Producing silicon and semiconductors for modern electronic equipment is even more energy consuming: 230 to 235 MJ per 1 kilogram of silicon, and about 3,000 MJ per kilogram of semiconductors.[67] This is much higher, compared to many other materials, e.g. production of iron from iron ore requires 20-25 MJ of energy, glass (from sand, etc.) - 18-35 MJ, steel (from iron) - 20-50 MJ, paper (from timber) - 25-50 MJ per kilogram.[68]
Incineration of plastics
Controlled high-temperature incineration, above 850C for two seconds,[69] performed with selective additional heating, breaks down toxic dioxins and furans from burning plastic, and is widely used in municipal solid waste incineration.[69] Municipal solid waste incinerators also normally include flue gas treatments to reduce pollutants further.[69] This is needed because uncontrolled incineration of plastic produces polychlorinated dibenzo-p-dioxins, a carcinogen (cancer causing chemical). The problem occurs as the heat content of the waste stream varies.[70] Open-air burning of plastic occurs at lower temperatures, and normally releases such toxic fumes.
Pyrolytic disposal
Plastics can be pyrolyzed into hydrocarbon fuels, since plastics have hydrogen and carbon. One kilogram of waste plastic produces roughly a liter of hydrocarbon.[71]
Recycling
Thermoplastics can be remelted and reused, and thermoset plastics can be ground up and used as filler, although the purity of the material tends to degrade with each reuse cycle. There are methods by which plastics can be broken back down to a feedstock state.
The greatest challenge to the recycling of plastics is the difficulty of automating the sorting of plastic wastes, making it labor-intensive. Typically, workers sort the plastic by looking at the resin identification code, although common containers like soda bottles can be sorted from memory. Typically, the caps for PETE bottles are made from a different kind of plastic which is not recyclable, which presents additional problems to the automated sorting process. Other recyclable materials such as metals are easier to process mechanically. However, new processes of mechanical sorting are being developed to increase capacity and efficiency of plastic recycling.
While containers are usually made from a single type and color of plastic, making them relatively easy to be sorted, a consumer product like a cellular phone may have many small parts consisting of over a dozen different types and colors of plastics. In such cases, the resources it would take to separate the plastics far exceed their value and the item is discarded. However, developments are taking place in the field of active disassembly, which may result in more consumer product components being re-used or recycled. Recycling certain types of plastics can be unprofitable, as well. For example, polystyrene is rarely recycled because it is usually not cost effective. These unrecycled wastes are typically disposed of in landfills, incinerated or used to produce electricity at waste-to-energy plants.
A first success in recycling of plastics is Vinyloop, a recycling process and an approach of the industry to separate PVC from other materials through a process of dissolution, filtration and separation of contaminations. A solvent is used in a closed loop to elute PVC from the waste. This makes it possible to recycle composite structure PVC waste which normally is being incinerated or put in a landfill. Vinyloop-based recycled PVC's primary energy demand is 46 percent lower than conventional produced PVC. The global warming potential is 39 percent lower. This is why the use of recycled material leads to a significant better ecological footprint.[72] This process was used after the Olympic Games in London 2012. Parts of temporary Buildings like the Water Polo Arena or the Royal Artillery Barracks were recycled. This way, the PVC Policy could be fulfilled which says that no PVC waste should be left after the games.[73]
In 1988, to assist recycling of disposable items, the Plastic Bottle Institute of the Society of the Plastics Industry devised a now-familiar scheme to mark plastic bottles by plastic type. A plastic container using this scheme is marked with a triangle of three "chasing arrows", which encloses a number giving the plastic type:

![]()





- PET (PETE), polyethylene terephthalate
- HDPE, high-density polyethylene
- PVC, polyvinyl chloride
- LDPE, low-density polyethylene,
- PP, polypropylene
- PS, polystyrene
- Other types of plastics (see list, below)
See also
- Conductive polymer
- Corn construction
- Molding (process)
- Films
- Light activated resin
- Nurdle
- Organic light emitting diode
- Plastics engineering
- Plastics extrusion
- Plastic film
- Plastic recycling
- Plasticulture
- Progressive bag alliance
- Roll-to-roll processing
- Self-healing plastic
- Thermoforming
- Timeline of materials technology
References
- ↑ "Terminology for biorelated polymers and applications (IUPAC Recommendations 2012)" (PDF). Pure and Applied Chemistry 84 (2): 377–410. 2012. doi:10.1351/PAC-REC-10-12-04.
- ↑ Life cycle of a plastic product. Americanchemistry.com. Retrieved on 2011-07-01.
- 1 2 3 4 5 6 7 8 9 10 11 12 Andrady AL, Neal MA (July 2009). "Applications and societal benefits of plastics". Philos. Trans. R. Soc. Lond., B, Biol. Sci. 364 (1526): 1977–84. doi:10.1098/rstb.2008.0304. PMC 2873019. PMID 19528050.
- ↑ American Chemical Society National Historic Chemical Landmarks. "Bakelite: The World’s First Synthetic Plastic". Retrieved 23 February 2015.
- ↑ Fantastic Recycled Plastic: 30 Clever Creations to Spark Your Imagination, by David Edgar, Robin A. Edgar, p11
- ↑ Polymer Chemistry: Introduction to an Indispensable Science, by David M. Teegarden, pp.58-59
- ↑ Plastikos, Henry George Liddell, Robert Scott, A Greek-English Lexicon, at Perseus. Perseus.tufts.edu. Retrieved on 2011-07-01.
- ↑ Plastic, Online Etymology Dictionary. Etymonline.com. Retrieved on 2011-07-01.
- ↑ UK Patent office (1857). Patents for inventions. UK Patent office. p. 255.
- ↑ Stephen Fenichell, Plastic: The Making of a Synthetic Century, HarperBusiness, 1996, ISBN 0-88730-732-9 p. 17
- ↑ "Dictionary – Definition of celluloid". Websters-online-dictionary.org. Retrieved 2011-10-26.
- 1 2 Christel Trimborn (August 2004). "Jewelry Stone Make of Milk". GZ Art+Design. Retrieved 2010-05-17.
- ↑ Trimborn, Christel (August 2004). "Jewelry Stone Make of Milk". GZ Art+Design.
- 1 2 Thompson RC, Swan SH, Moore CJ, vom Saal FS (July 2009). "Our plastic age". Philos. Trans. R. Soc. Lond., B, Biol. Sci. 364 (1526): 1973–6. doi:10.1098/rstb.2009.0054. PMC 2874019. PMID 19528049.
- ↑ "Historical Overview and Industrial Development". International Furan Chemicals, Inc. Retrieved 4 May 2014.
- ↑ Hans-Georg Elias "Plastics, General Survey" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a20_543
- 1 2 3 Teuten EL, Saquing JM, Knappe DR, et al. (July 2009). "Transport and release of chemicals from plastics to the environment and to wildlife". Philos. Trans. R. Soc. Lond., B, Biol. Sci. 364 (1526): 2027–45. doi:10.1098/rstb.2008.0284. PMC 2873017. PMID 19528054.
- 1 2 Seymour, Raymond Benedict; Deaning, Rudolph D. (1987). History of Polymeric Composites. VSP. p. 374.
- ↑ Classification of Plastics. Dwb.unl.edu. Retrieved on 2011-07-01.
- ↑ Composition and Types of Plastic Inforplease website
- ↑ Biodegradation of plastic bottles made from Biopol in an aquatic ecosystem under in situ conditions, accessed March 2009 (login required). Springerlink.com. Retrieved on 2011-07-01.
- ↑ National Non-Food Crops Centre. Biochemical Opportunities in the UK, NNFCC 08-008
- ↑ National Non-Food Crops Centre. NNFCC Renewable Polymers Factsheet: Bioplastics
- ↑ Plastics News. Plastics News. Retrieved on 2011-07-01.
- ↑ Watson, Peter. A Terrible Beauty (also published as Modern Mind: An intellectual history of the 20th century). London: Weidenfeld & Nicolson Ltd (imprint of Orion Books). 2001
- ↑ Jezek, Geno. "What is Vinyl?". Retrieved January 2011.
- ↑ "Polyvinyl chloride". Plasticsusa.com. Retrieved January 2011.
- ↑ Salocks, Charles and Kaley, Karlyn Black (Revised 4 February 2004). "Technical Support Document: Toxicology of Hydrogen Chloride" (PDF). California EPA, Office of Environmental Health Hazard Assessment. p. 8. Retrieved January 2011. Check date values in:
|date=(help) - ↑ "How can I preserve my family photographs for my grandchildren?". The Library of Congress Preservation FAQs. LoC. Retrieved January 2011.
- ↑ Kinnane, Adrian (2002). DuPont: From the banks of the Brandywine to miracles of science. Baltimore, Md.: Johns Hopkins University Press. pp. 116–125. ISBN 0-8018-7059-3.
- ↑ Periodic Table of Polymers Dr Robin Kent – Tangram Technology Ltd.
- ↑ Heeger, A. J.; Schrieffer, J. R.; Su, W. -P.; Su, W. (1988). "Solitons in conducting polymers". Reviews of Modern Physics 60 (3): 781–850. Bibcode:1988RvMP...60..781H. doi:10.1103/RevModPhys.60.781.
- 1 2 3 McRandle, P.W. (March–April 2004). "Plastic Water Bottles". National Geographic. Retrieved 2007-11-13.
- ↑ Chun Z. Yang, Stuart I. Yaniger, V Craig. Jordan, Daniel J. Klein, George D. Bittner. Most Plastic Products Release Estrogenic Chemicals: A Potential Health Problem That Can Be Solved. Environmental Health Perspectives.
- ↑ Perinatal Exposure to Low Doses of Bisphenol A Affects Body Weight, Patterns of Estrous Cyclicity, and Plasma LH Levels, accessed March 2009. Ehponline.org. Retrieved on 2011-07-01.
- ↑ Alonso-Magdalena, Paloma; Morimoto, Sumiko; Ripoll, Cristina; Fuentes, Esther; Nadal, Angel (January 2006). "The Estrogenic Effect of Bisphenol A Disrupts Pancreatic β-Cell Function In Vivo and Induces Insulin Resistance". Environmental Health Perspectives 114 (1): 106–112. doi:10.1289/ehp.8451. PMC 1332664. PMID 16393666.
- ↑ Andrew Zajac FDA issues BPA guidelines, Los Angeles Times, January 16, 2010
- ↑ Lisa Wade McCormick More Kids' Products Found Containing Unsafe Chemicals, ConsumerAffairs.com, October 30, 2009
- ↑ Kinoshita, S.; Kageyama, S., Iba, K., Yamada, Y. and Okada, H. (1975). "Utilization of a cyclic dimer and linear oligomers of e-aminocaproic acid by Achromobacter guttatus". Agricultural & Biological Chemistry 39 (6): 1219–23. doi:10.1271/bbb1961.39.1219. ISSN 0002-1369.
- ↑ Yutaka Tokiwa; Buenaventurada P. Calabia; Charles U. Ugwu; Seiichi Aiba (September 2009). "Biodegradability of Plastics". International Journal of Molecular Science 9 (9): 3722–3742. doi:10.3390/ijms10093722. PMC 2769161. PMID 19865515.
- ↑ Jonathan R. Russell; Jeffrey Huang; Scott A. Strobel (September 2011). "Biodegradation of Polyester Polyurethane by Endophytic Fungi". Applied environmental Microbiology 77: 6076–6084. doi:10.1128/aem.00521-11. PMC 3165411. PMID 21764951.
- ↑ "Biodegradation of Polyester Polyurethane by Endophytic Fungi". Applied and Environmental Microbiology. July 2011.
- ↑ Link PDF
- ↑ Immortal Polystyrene Foam Meets its Enemy | LiveScience
- ↑ Ward, PG; Goff, M; Donner, M; Kaminsky, W; O'Connor, KE. (2006). "A two step chemo-biotechnological conversion of polystyrene to a biodegradable thermoplastic". Environmental Science and Technology 40 (7): 2433–7. doi:10.1021/es0517668. PMID 16649270.
- ↑ Cacciari I; Quatrini P; Zirletta G; Mincione E; Vinciguerra V; Lupattelli P; Giovannozzi Sermanni G (1993). "Isotactic polypropylene biodegradation by a microbial community: physicochemical characterization of metabolites produced". Applied and Environmental Microbiology 59.
- ↑ Ishtiaq Ali, Muhammad (2011). Microbial degradation of polyvinyl chloride plastics (PDF) (Ph.D.). Quaid-i-Azam University. pp. 45–46.
- 1 2 Ishtiaq Ali, Muhammad (2011). Microbial degradation of polyvinyl chloride plastics (PDF) (Ph.D.). Quaid-i-Azam University. p. 76.
- ↑ Ishtiaq Ali, Muhammad (2011). Microbial degradation of polyvinyl chloride plastics (PDF) (Ph.D.). Quaid-i-Azam University. p. 122.
- ↑ Yutaka Tokiwa; Buenaventurada P. Calabia; Seiichi Aiba (September 2009). "Biodegradability of Plastics". International Journal of Molecular Science 10 (9): 3722–3744. doi:10.3390/ijms10093722. PMC 2769161. PMID 19865515.
- ↑ "CanadaWorld – WCI student isolates microbe that lunches on plastic bags". The Record.com.
- ↑ Hadad D; Geresh S; Sivan A (2005). "Biodegradation of polyethylene by the thermophilic bacterium Brevibacillus borstelensis". journal of applied microbiology 98: 1093–100. doi:10.1111/j.1365-2672.2005.02553.x. PMID 15836478.
- ↑ Trudy E. Bell (2007). "Preventing "Sick" Spaceships".
- ↑ Francesca Cappitelli; Claudia Sorlini (2008). "Microorganisms Attack Synthetic Polymers in Items Representing Our Cultural Heritage". Applied Environmental Microbiology 74 (3): 564–569. doi:10.1128/AEM.01768-07. PMC 2227722. PMID 18065627.
- ↑ Gwyneth Dickey Zaikab (March 2011). "Marine microbes digest plastic".
- ↑ Chetna Sharon; Madhuri Sharon (2012). "Studies on Biodegradation of Polyethylene terephthalate: A synthetic polymer" (PDF). Journal of Microbiology and Biotechnology Research. pp. 248–257.
- ↑ "Fungus eats CD". Nature. 2001.
- ↑ "Fungus 'eats' CDs". BBC. June 2001.
- ↑ Gusse AC; Miller PD; Volk TJ (July 2006). "White-rot fungi demonstrate first biodegradation of phenolic resin". Environmental Science and Technology 40 (13): 4196–9. doi:10.1021/es060408h. PMID 16856735.
- ↑ Cappitelli F; Principi P; Sorlini C. (Aug 2006). "Biodeterioration of modern materials in contemporary collections: can biotechnology help?". Trends in Biotechnology 24: 350–4. doi:10.1016/j.tibtech.2006.06.001. PMID 16782219.
- ↑ Andrea Rinaldi (November 7, 2006). "Saving a fragile legacy. Biotechnology and microbiology are increasingly used to preserve and restore the worlds cultural heritage". EMBO Reports 7 (11): 1075–1079. doi:10.1038/sj.embor.7400844. PMC 1679785. PMID 17077862.
- ↑ Alan Weisman, "The World Without Us," HarperCollins Canada, 2010 ISBN 1443400084.
- ↑ What's a Nurdle? – Ocean Defenders – the weblog. Weblog.greenpeace.org. Retrieved on 2011-11-03.
- 1 2 3 Barnes DK, Galgani F, Thompson RC, Barlaz M (July 2009). "Accumulation and fragmentation of plastic debris in global environments". Philos. Trans. R. Soc. Lond., B, Biol. Sci. 364 (1526): 1985–98. doi:10.1098/rstb.2008.0205. PMC 2873009. PMID 19528051.
- ↑ EPA. (2012). Landfilling.
- ↑ Is Biodegradability a Desirable Attribute for Discarded Solid Waste? Perspectives from a National Landfill Greenhouse Gas Inventory Model. Environ. Sci. Technol.
- ↑ http://www.lowtechmagazine.com/2009/06/embodied-energy-of-digital-technology.html The monster footprint of digital technology, Low Tech magazine, 2009. Retrieved on 2012-11-11.
- ↑ http://www.lowtechmagazine.com/what-is-the-embodied-energy-of-materials.html How much energy does it take (on average) to produce 1 kilogram of the following materials? Low Tech magazine, 2009. Retrieved on 2012-11-11.
- 1 2 3 See incineration for references - except that there is no reference on the incineration page for the need to reach 850 C
- ↑ "Plastics and Health Risks". Annual Review of Public Health 31: 179–194. doi:10.1146/annurev.publhealth.012809.103714.
- ↑ The Hindu-Dec 2005. Hindu.com (2005-12-19). Retrieved on 2011-07-01.
- ↑
- ↑ "Implementation of the PVC policy". The London Organising Committee of the Olympic Games and Paralympic Games Limited. Retrieved October 24, 2012.
- ↑ SPI Resin Identification Code – Guide to Correct Use. plasticsindustry.org
- Substantial parts of this text originated from An Introduction To Plastics v1.0 / 1 March 2001 / greg goebel / public domain.
External links
| Wikimedia Commons has media related to Plastics. |
- J. Harry Dubois Collection on the History of Plastics, ca. 1900–1975 Archives Center, National Museum of American History, Smithsonian Institution.
- Material Properties of Plastics – Mechanical, Thermal & Electrical Properties
- List of over 600 plastics
- Plastics Historical Society
- History of plastics, Society of the Plastics Industry
- What Are Plastics Made Of? January 1943 Popular Science
- "A brief history of plastics, natural and synthetic", from BBC Magazine
- Scientists have announced a new rock type: plastiglomerate
- Timeline of important milestone of plastic injection moulding and plastics
| ||||||||||||||||||||||||||
|