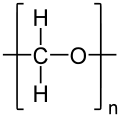
Polyoxymethylene
 | |
 | |
| Names | |
|---|---|
| Other names
Poly(oxymethylene) glycol; Polymethylene glycol | |
| Identifiers | |
| 9002-81-7 | |
| Properties | |
| (CH2O)n | |
| Molar mass | Variable |
| Appearance | Colorless solid |
| Density | 1.41–1.42 g/cm3 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |

Polyoxymethylene (POM), also known as acetal,[1] polyacetal and polyformaldehyde, is an engineering thermoplastic used in precision parts requiring high stiffness, low friction and excellent dimensional stability. As with many other synthetic polymers, it is produced by different chemical firms with slightly different formulas and sold variously by such names as Delrin, Celcon, Ramtal, Duracon, Kepital and Hostaform.
Typical applications for injection-molded POM include high performance engineering components such as small gear wheels, ball bearings, ski bindings, fasteners, knife handles, and lock systems. The material is widely used in the automotive and consumer electronics industry.
Development
Polyoxymethylene was discovered by Hermann Staudinger, a German chemist who received the 1953 Nobel Prize in Chemistry. He had studied the polymerization and structure of POM in the 1920s while researching macromolecules, which he characterized as polymers. Due to problems with thermal stability, POM was not commercialized at that time.
Around 1952 research chemists at DuPont synthesized a version of POM, and in 1956 the company filed for patent protection of the homopolymer. DuPont credits R. N. MacDonald as the inventor of high molecular weight POM.[2] Patents by MacDonald and coworkers describe the preparation of high molecular weight hemiacetal (~O-CH2OH) terminated POM,[3] but these lack sufficient thermal stability to be commercially viable. The inventor of a heat-stable (and therefore useful) POM homopolymer was Dal Nagore,[4] who discovered that reacting the hemiacetal ends with acetic anhydride converts the readily depolymerizable hemiacetal into a thermally stable, melt processable plastic.
DuPont completed construction of a plant to produce its own version of acetal resin, named Delrin at Parkersburg, West Virginia, in 1960. Also in 1960, Celanese completed its own research. Shortly thereafter, in a limited partnership with the Frankfurt firm Hoechst AG, a factory was built in Kelsterbach, Hessen; from there, Celcon was produced starting in 1962, with Hostaform joining it a year later. Both remain in production under the auspices of Celanese, and are sold as parts of a product group now called Hostaform/Celcon POM.
Properties
POM is characterized by its high strength, hardness and rigidity to −40 °C. POM is intrinsically opaque white, due to its high crystalline composition, but it is available in all colors. POM has a density of ρ = 1.410–1.420 g/cm3.[5]
POM homopolymer is a semi-crystalline polymer (75–85% crystalline) with a melting point of 175 °C (347 °F). The POM copolymer has a slightly lower melting point of 162–173 °C.
POM is a tough material with a very low coefficient of friction. However, it is susceptible to polymer degradation catalyzed by acids, which is why it is stabilized - both the homopolymer and the copolymer have chain end groups (introduced via end capping) that resist depolymerization. With the copolymer, the second unit is normally a C2 (ethylene glycol) or C4 (1,4-butanediol) unit, which is introduced via its cyclic acetal (which can be made from the diol and formaldehyde) or cyclic ether (e.g. ethylene oxide). These units resist chain cleavage, because the O-linkage is now no longer an acetal group, but an ether linkage, which is stable to hydrolysis. POM is sensitive to oxidation, and an anti-oxidant is normally added to molding grades of the material.
POM advantages:
- High abrasion resistance
- Low coefficient of friction
- High heat resistance
- Good electrical and dielectric properties
- Low water absorption
Production
Different manufacturing processes are used to produce the homopolymer and copolymer versions of POM.
Homopolymer
To make polyoxymethylene homopolymer, anhydrous formaldehyde must be generated. The principal method is by reaction of the aqueous formaldehyde with an alcohol to create a hemiformal, dehydration of the hemiformal/water mixture (either by extraction or vacuum distillation) and release of the formaldehyde by heating the hemiformal. The formaldehyde is then polymerized by anionic catalysis and the resulting polymer stabilized by reaction with acetic anhydride. Due to the manufacturing process, large diameter cross sections may have pronounced centerline porosity.[6] A typical example is DuPont’s Delrin.
Copolymer
To make polyoxymethylene copolymer, formaldehyde is generally converted to trioxane (specifically 1,3,5-trioxane, also known as trioxin). This is done by acid catalysis (either sulfuric acid or acidic ion exchange resins) followed by purification of the trioxane by distillation and/or extraction to remove water and other active hydrogen containing impurities. Typical copolymers are Hostaform from Celanese and Ultraform from BASF.
The co-monomer is typically dioxolane but ethylene oxide can also be used. Dioxolane is formed by reaction of ethylene glycol with aqueous formaldehyde over an acid catalyst. Other diols can also be used.
Trioxane and Dioxolane are polymerized using an acid catalyst, often boron trifluoride etherate, BF3 OEt2. The polymerization can take place in a non-polar solvent (in which case the polymer forms as a slurry) or in neat trioxane (e.g. in an extruder). After polymerization, the acidic catalyst must be deactivated and the polymer stabilized by melt or solution hydrolysis to remove unstable end groups.
Stable polymer is melt compounded, adding thermal and oxidative stabilizers and optionally lubricants and miscellaneous fillers.
Fabrication
POM is supplied in a granulated form and can be formed into the desired shape by applying heat and pressure. The two most common forming methods employed are injection molding and extrusion. Rotational molding and blow molding are also possible.
Typical applications for injection-molded POM include high performance engineering components (e.g. gear wheels, ski bindings, yoyos, fasteners, lock systems) and the material is widely used in the automotive and consumer electronics industry. There are special grades that offer higher mechanical toughness, stiffness or low friction/ wear properties.
POM is commonly extruded as continuous lengths of round or rectangular section. These sections can be cut to length and sold as bar or sheet stock for machining.
Machining
When supplied as extruded bar or sheet, POM may be machined using traditional methods such as turning, milling, drilling etc. These techniques are best employed where production economics do not merit the expense of melt processing. The material is free-cutting, but does require sharp tools with a high clearance angle. The use of soluble cutting lubricant is not necessary, but is recommended.
POM sheets can be cut cleanly and accurately using an infrared laser, such as in a CO2 laser cutter.
Because the material lacks the rigidity of most metals, care should be taken to use light clamping forces and sufficient support for the work piece.
Machined POM can be dimensionally unstable, especially with parts that have large variations in wall thicknesses. It is recommended that such features be ‘designed-out’ e.g. by adding fillets or strengthening ribs. Annealing of pre-machined parts before final finishing is an alternative. A rule-of-thumb is that in general, small components machined in POM suffer from less warping.
Bonding
POM is typically very difficult to bond. Special processes and treatments have been developed to improve bonding. Typically these processes involve surface etching, flame treatment or mechanical abrasion.
Typical etching processes involve chromic acid at elevated temperatures. DuPont uses a patented process for treating acetal homopolymer called satinizing that creates a surface roughness sufficient for micromechanical interlocking. There are also processes involving oxygen plasma and corona discharge.[7][8]
Once the surface is prepared, a number of adhesives can be used for bonding. These include epoxies, polyurethanes, and cyanoacrylates. Epoxies have shown 150–500 psi (1,000–3,400 kPa) shear strength on mechanically abraded surfaces and 500–1,000 psi (3,400–6,900 kPa) on chemically treated surfaces. Cyanoacrylates are useful for bonding to metal, leather, rubber and other plastics.
Solvent welding is typically unsuccessful on acetal polymers, due to the excellent solvent resistance of acetal.
Thermal welding through various methods has been used successfully on both homopolymer and copolymer.
Usage
- Mechanical gears, sliding and guiding elements, housing parts, springs, chains, screws, nuts, fan wheels, pump parts, valve bodies.
- Electrical Engineering: insulators, bobbins, connectors, parts for electronic devices such as televisions, telephones, etc.
- Vehicle: Fuel sender unit, Light stock (including shifter for light, turn signal), power windows, door lock systems, articulated shells.
- Model: Model Railway parts, such as trucks (bogies) and hand rails (handle bars). POM is tougher than ABS, comes in bright translucent colors, and is not paintable.
- Hobbies: Radio-controlled helicopter Main gear, landing skid, yo-yos, vaping drip tips, etc.
- Medical: insulin pen, Metered dose inhalers (MDI)
- Food industry: Food and Drug Administration has approved some grades of POM for milk pumps, coffee spigots, filter housings and food conveyors.[9]
- Furniture: hardware, locks, handles, hinges.
- Construction: Structural Glass - pod holder for point
- Packaging: aerosol cans, vehicle tanks.
- Sports: Paintball accessories. It is often used for machined parts of paintball markers that do not require the strength of aluminum, such as handles and reciprocating bolts. POM is also used in airsoft guns to reduce piston noise.
- Longboarding: Puck material for slide gloves help the rider touch the road and lean on their hand to slow down, stop, or perform tricks.
- Clothing: zippers.
- Music: picks, Irish flutes, Bagpipes, Practice chanters, harpsichord plectra, tuba mouthpieces, tips of some drum sticks.[10]
- Dining: Fully automatic coffee brewers; knife handles (particularly folding knives)
- Horology: Watch bracelets (e.g. IWC Porsche Design 3701)
- Electronic Cigarettes (e.g. Drip Tips)
Degradation

Acetal resins are sensitive to acid hydrolysis and oxidation by agents such as mineral acids and chlorine. POM homopolymer is also susceptible to alkaline attack and is more susceptible to degradation in hot water. Thus low levels of chlorine in potable water supplies (1–3 ppm) can be sufficient to cause stress corrosion cracking, a problem experienced in both the USA and Europe in domestic and commercial water supply systems. Defective mouldings are most sensitive to cracking, but normal mouldings can succumb if the water is hot. Both POM homopolymer and copolymer are stabilized to mitigate these types of degradation.
Widespread failure of acetal mouldings in potable and hot water supplies resulted in one of the largest class actions in the USA when acetal plumbing fittings cracked and caused flooding of homes, a problem exacerbated by similar problems with polybutylene pipework. The acetal fittings tended to fail first, followed by the pipework.
In chemistry applications, whilst the polymer is often suitable for the majority of glassware work, it can succumb to catastrophic failure. An example of this would be using the polymer clips on hot areas of the glassware (such as a flask to column, column to head or head to condenser joint during distillation). As the polymer is sensitive to both chlorine and acid hydrolysis, it may perform very poorly when exposed to the reactive gases, particularly hydrogen chloride. Failures in this latter instance can occur with seemingly unimportant exposures from well sealed joints, and do so without warning and rapidly (the component will split or fall apart). This can be a significant health hazard as the glass may open or smash. Here, PTFE or a high grade stainless steel may be a more appropriate choice.
See also
References
- ↑ "MatWeb:acetal".
- ↑ http://uk.news.dupont.com/site/contenu.asp?idtri=624&idcontenu=56221
- ↑ US 2768994
- ↑ US 2998409
- ↑ Ticona MSDS for Hostaform <http://tools.ticona.com/tools/mcbasei/product-tools.php?sPolymer=POM&sProduct=HOSTAFORM/Celcon
- ↑
- ↑ BASF Ultraform product information
- ↑ Snogren, R. C. (1974). Handbook of Surface Preparation. New York: Palmerton Publishing Co.
- ↑ "Acetal Plastic Sheet, Rod, Tube and Accessories". Interstate Plastics. Interstate Plastics. Retrieved September 2015.
- ↑ Murphy, Joe. "The Loud Buzzer". unknown. Retrieved 2012-03-17.
External links
- "Acetal". Machine Design. November 15, 2002. Retrieved August 2015.
- "Acetal (POM) Engineering Property Data". MatWeb. Retrieved August 2015.
- Michael Sepe (September 2012). "How Do You Like Your Acetal: Homopolymer or Copolymer?". Plastics Technology. Retrieved August 2015.