Polybenzimidazole fiber
| | |
| Identifiers | |
|---|---|
| 32075-68-6 | |
| Properties | |
| (C20H12N4)n | |
| Molar mass | Variable |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Polybenzimidazole (PBI, short for poly[2,2’-(m-phenylen)-5,5’-bisbenzimidazole]) fiber is a synthetic fiber with a very high melting point. It has exceptional thermal and chemical stability and does not readily ignite. It was first discovered by American polymer chemist Carl Shipp Marvel in the pursuit of new materials with superior stability, retention of stiffness, toughness at elevated temperature. Due to its high stability, polybenzimidazole is used to fabricate high-performance protective apparel such as firefighter’s gear, astronaut space suits, high temperature protective gloves, welders’ apparel and aircraft wall fabrics. In recent years, polybenzimidazole found its application as a membrane in fuel cells.
History
Discovery
Brinker and Robinson invented the first aliphatic polybenzimidazoles in 1949.[1] However the discovery of aromatic polybenzimidazole which show excellent physical and chemical properties was generally credited to Carl Shipp Marvel in the 1950s.[2] The materials Laboratory of Wright Patterson Air Force Base approached Marvel. They were looking for materials suitable for drogue parachutes which could tolerate short-time mechanical stress. However, the thermal resistance of all known filaments at that time was completely inadequate. The original search concentrated on aromatic condensation polymers but the amide linkage proved to be weak link for the aim of maximal thermal stability of the polymer, whereas Marvel’s research focused on condensation polymers with aromatic and heteroaromatic repeating units. This progressively led to the discovery of polybenzimidazole.
Development

Its development history can be summarized in the following list:[3]
- In 1961, polybenzimidazole was developed by H. Vogel and C.S. Marvel with anticipation that the polymers would have exceptional thermal and oxidative stability.
- Subsequently in 1963, NASA and the Air Force Materials Lab sponsored considerable work with polybenimidazole for aerospace and defense applications as a non-flammable and thermally stable textile fiber.
- In 1969, the United States Air Force selected polybenzimidazole (PBI) for its superior thermal protective performance after a 1967 fire aboard the Apollo 1 spacecraft killed three astronauts.
- In the 1970s, NASA continued to use PBI as part of the astronauts’ clothing on Apollo, Skylab and numerous space shuttle flights.
- When the Sky lab fell to the earth, the part that survived the re-entry was coated in PBI and thus did not burn up.
- 1980s – PBI was introduced to the fire service, and through Project Fires an outer shell for turnout gear was developed. PBI Gold® fabric was born, consisting of 40% PBI/60% para-aramid. Previous to this, combinations of Nomex, leather, and Kevlar materials were used in the US.
- Leather and rubber fire gear left “vital” areas exposed.
- PBI gear is a full suit and looks like a snow suit (suspender pants) with a winter coat.
- 1983 – A unique production plant goes on-line and PBI fibers become commercially available.
- 1990s Short-cut PBI fibers are introduced for use in automotive braking systems. PBI staple fiber enters the aircraft market for seat fire blocking layers.
- 1992 Lightweight PBI fabrics are developed for flame-resistant workwear for electric utility and petrochemical applications.
- 1994 PBI Gold fabric is engineered in black and was specified by FDNY.
- 2001 – After the terrorist attacks on September 11, many of the 343 fire fighters killed were only identifiable by their PBI Turnout Gear.
- 2003 PBI Matrix® was commercialized and introduced as the next-generation PBI for firefighter turnout gear.
Properties
General physical properties
For the physical properties, PBI are usually yellow to brown solid infusible up to 400 °C or higher.[4] The solubility of PBI is controversial. Because although most of the linear PBI are partly or entirely dissolved in strong protonic acids for instance, sulfuric acid or methanesulfonic acid, contradictory observations of solubities have been recorded among such weaker acids as formic acid, and in non-acidic media, such as the aprotic amide-type solvents and dimethyl sulfoxide. For example, one type pf PBI prepared in phosphoric acid was found by Iwakura et al.[5] to be partially soluble in formic acid, but completely soluble in dimethyl sulfoxide and dimethylacetamide, whereas Varma and Veena[6] reported the same polymer type to dissolve completely in formic acid, yet only partially in dimethyl sulfoxide or dimethylacetamide.
Thermal stability
Imidazole derivatives are known to be stable compounds. Many of them are resistant to the most drastic treatments with acids and bases and not easily oxidized. The high melting point and high stability at over 400 degree suggests a polymer with benzimidazole as repeating unit may also show high heat stability. Polybenzimidazole and its aromatic derivatives can withstand temperatures in excess of about 500 degree without softening and degrading. The polymer synthesized from isophthalic acid and 3,3'-diaminobenzidine is not melted by exposure to a temperature of 770 degree and loses only 30% of its weight after exposure to high temperature up to 900 degree for several hours.[7] This proves a high thermal stability for PBI.
Flame resistance
A property of a material needed to be considered before putting it into application is flammability, which demonstrates how easily one material can ignite and combust under the realistic operating conditions. This may affect its application in varied areas, such as in construction, plant design, and interior decoration. The quantitative assessment of flammability is based on limiting oxygen index(LOI), i.e., the minimum oxygen concentration at which a given sample can be induced to burn permit a comparison of flammability. Data shows that PBI are highly flame resistant material compared to common polymers.[8]
Moisture regain
Another particular property of PBI, the remarkable moisture regain is useful in protective clothing which make the clothing quite comfortable to wear in sharp contrast to other synthetic polymers. The moisture regain ability of PBI compares favorably with cotton which regains 13% of moisture with a 16% of cotton.[9]
Synthesis
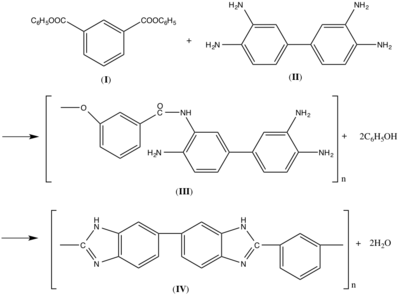
The preparation of PBI(IV) can be achieved by condensation reaction of diphenyl isophthalate (I) and 3,3’,4,4’-tetraaminodiphenyl (II) (Figure 1). The spontaneous cyclization of the intermediately formed animo-amide (III) to PBI (IV) provided a much more stable amide linkage.
This synthetic method was first used in the lab and later developed into a two step process. In a typical condition, starting materials were heated at 270 degree for 1.5 h to form the PBI prepolymer and later the prepolymer was heated at 360 degree for another 1 h to form the final commercial-grade product. The reason for the second step is due to the formation of the by-product phenol and water in the first step creating voluminous foam,[10] which leads to the volume expansion of several times of the original. This is the issue that must be considered by the industrial manufacturers.
This foam can be reduced by conducting the polycondensation at a high temperature around 200 °C and under the pressure of 2.1-4.2 MPa.[11] The foam can also be controlled by adding high boiling point liquids such as diphenylether or cetane to the polycondesation. The boiling point can make the liquid stay in the first stage of polycondesation but evaporate in the second stage of solid condensation. The disadvantage of this method is there are still some liquid remain in PBI and it is hard to remove them completely.[12]
While changing the tetramine and acid, a number of different aromatic poly benzimidazoles have been synthesized. The following table (Table 1)[13] lists out some of the combination possibilities that have been synthesized in the literature. Some of the combination have actually been translated into fibers on a small scale. However, the only significant progress have been made to date is PBI.

| R(Teraamine) | R'(acid)) |
|---|---|
| Benzene | Benzene |
| Diphenyl | Diphenyl |
| Diphenylether | Diphenylether |
| Diphenylsulfone | Naphthalene |
| Naphthalene | Pyridine |
| Pyridine | Anthraquinone |
| Anthraquinone | Ferrocene |
| Anthracene | |
The most common form of PBI used in industry is the fiber form. The fiber process following polymerization is shown in the figure. The polymer is made into solution using dimethylacetamide as solvent. The solution is filtered and converted into fiber using a high temperature dry-spinning process. The fiber is subsequently drawn at elevated temperature to get desired mechanical properties. It is then sulfonated and made into staple using conventional crimping and cutting techniques.

Applications
Before the 1980s, major applications of PBI are for fire-blocking, thermal protective apparel and reverse osmosis membranes. Its applications became various by the 1990s with the fact that the molded PBI parts and microporous membranes were developed.
Protective apparel
The properties such as thermal stability, flame resistant, and moisture regain of PBI and its conventional textile processing character enable it to be processed on conventional staple fiber textile equipment. These characters lead to one of the most important applications of PBI is for protective apparel. PBI filaments were fabricated into protective gears like firefighter’s gear, astronauts suits. PBI filaments are dry spun from dimethylacetamide containing lithium chloride. After washing and drying the resulting yarn is golden brown.

PBI fiber is an excellent candidate for applications in severe environments due to its combination of thermal, chemical and textile properties. Flame and thermal resistance are the critical properties of protective apparel. This kind of apparel applications includes firefighter’s protective apparel, astronaut’s suits,[14] aluminized crash rescued gear, industrial worker’s apparel, and suits for racing car drivers.[15]

The problem of PBI protective apparel is that it keeps heat out but it also keeps the heat in, too. Thus, by the time the firefighters feel heat and pain, it is too late and they will get burnt or killed since it is hard for the heat to penetrate the suit and be released into the air. There are still some major fire departments have not yet switched from old fire gear to PBI due to the fear of what PBI can cause. One example is the Chicago Fire Department, which still relies on the old type of rubber or leather coats.[16]
PBI membranes
PBI has been used as the membranes for various separation purposes. Traditionally, PBI was used semi-permeable membranes for electrodialysis, reverse osmosis or ultrafiltration.[17] Recently PBI is also used for gas separations.[18] due to its close chain packing since PBI has rigidity structure and strong hydrogen bonding. PBI membranes are dense, with very low gas permeability.To be proton conductive, PBI usually is doped with acid. The higher level of the acid doping, the more conductive PBI is. But one problem raised is the mechanical strength of PBI decreases at the same time. The optimum doping level is thus a compromise between these two effects. Thus, multiple methods such as ionic cross-linking, covlant cross-linking and composite membranes[19] have been researched to optimize the doping level at which PBI has an improved conductivity without sacrificing mechanical strength. Kerres et al.[20] recently have recently synthesized sulphonated partially fluorinated arylene main chain polymer. Their blend membranes with PBI demonstrate high level acid-doping levels with thermal and extended stability, high proton conductivities, less acid swelling, reasonable mechanical strength.

Molded PBI resin
PBI resin is molded via a sintering process that was jointly developed by Hoechst Celanese (North Carolina, USA) and Alpha Precision Plastics, Inc. (Houston, Texas, USA).[21] Molded PBI resin is an excellent candidate for high strength, low weight material. Since it has the highest compressive strength, 58 ksi, of any available, unfilled resin and other mechanical properties such as a tensile strength of 23 ksi, a flexural strength of 32 ksi, a ductile compressive failure mode and the relatively low density of 1.3 g/cm3.[22] Moreover, its thermal and electrical properties also make it a well known thermoplastic resin. The PBI resin comprises a recurring structural unit represented by the following figure.
According to the Composite Materials Research Group at the University of Wyoming, PBI resin parts maintain significant tensile properties and compressive strength to 700 °F (371 °C). PBI resin parts are also potential materials for the chemical process and oil recovery industries which have demands of thermal stability and chemical resistance. In these areas, PBI resin has been successfully applied in demanding sealing, for instance, valve seats, stem seals, hydraulic seals and backup rings. In the aerospace industry, PBI resin has high strength and short term high temperature resistance advantages. In the industrial sector, PBI resin's high dimensional stability as well as retention of electrical properties at high temperature make it used as a thermal and electrical insulator.[23]
Fuel cell electrolyte
Polybenzimidazole is able to be complexed by strong acids because of its basic character. Complexation by phosphoric acid makes it a proton conductive material.[24] This renders the possible application to high temperature fuel cells. Cell performance test show a good stability in performance for 200 h runs at 150 degree. Application in direct methanol fuel cells may be also of interest because of a better selectivity water/methanol compared to existing membranes. Wainright, Wang et al. reported that PBI doped with phosphoric acid was utilized as a high temperature fuel cell electrolyte.[25] The doped PBI high temperature fuel cell electrolyte has several advantages. The evevated temperature increases the kinetic rates of the fuel cell reactions. It also can reduce the problem of the catalyst poisoning by adsorbed carbon monoxide and it minimizes problems due to electrode flooding.[26] PBI/H3PO4 is conductive even in low relative humidity and it allows less crossover of the methanol at the same time.[27] These contribute PBI/H3PO4 to be superior to some traditional polymer electrolytes such as Nafion. Additionally, PBI/H3PO4 maintains good mechanical strength and toughness.[28] Its modulus is three order of magnitude greater than that of Nafion.[29] This means that the thinner films can be used, thus reducing ohmic loss.

Asbestos replacement
Previously, only asbestos can well perform in temperature gloves such as for foundries, aluminum extrusion, and metal treatment, while PBI trials have been developed and show adequately functions as asbestos. Moreover, a safety garment manufacturer reported that gloves containing PBI outlasted asbestos two to nine times with an effective cost.[30] Gloves containing PBI fibers are softer and more supple than those made of asbestos, offering,the worker greater mobility, and comfort even if the fabric becomes charred. Besides, PBI fiber avoids the chronic toxicity problems associated with asbestos because it processes on standard textile and glove fabricating equipment.[31] PBI also can also be a good substitute for asbestos in several areas of glass manufacturing.
Flue gas filtration
PBI’s chemical, thermal and physical properties demonstrate that it can be a promising material as a flue gas filter fabric for coal fired boilers. Few fabrics can survive in the acidic and high temperature environment encountered in coal fired boiler flue gas.[32] The filter bags also must be able to bear the abrasion from the periodic cleaning to remove accumulated dust. PBI fabric has a good abrasion resistance property. The acid and abrasion resistance and thermal stability properties make PBI a competitor for this application.
References
- ↑ "Patent on aliphatic polybenzimidazole". Retrieved 7 March 2014.
- ↑ Leonard, Nelson. "A Biographic Memoir of Carl Shipp Marvel" (PDF). National Academy of Sciences. Retrieved 13 February 2014.
- ↑ "PBI History". Retrieved 14 February 2014.
- ↑ Synthesis and degradation, rheology and extrusion. Berlin u.a.: Springer. 1982. ISBN 978-3-540-11774-2.
|first1=missing|last1=in Authors list (help) - ↑ Iwakura, Yoshio; Uno, Keikichi; Imai, Yoshio (June 1964). "Polyphenylenebenzimidazoles". Journal of Polymer Science Part A: General Papers 2 (6): 2605–2615. doi:10.1002/pol.1964.100020611.
- ↑ Varma, I. K.; Veena, (April 1976). "Effect of structure on properties of aromatic-aliphatic polybenzimidazoles". Journal of Polymer Science: Polymer Chemistry Edition 14 (4): 973–980. Bibcode:1976JPoSA..14..973V. doi:10.1002/pol.1976.170140417.
- ↑ Vogel, Herward; Marvel, C. S. (April 1961). "Polybenzimidazoles, new thermally stable polymers". Journal of Polymer Science 50 (154): 511–539. Bibcode:1961JPoSc..50..511V. doi:10.1002/pol.1961.1205015419.
- ↑ van Krevelen, Dirk W. (30 March 1972). "New developments in the field of flame-resistant fibres". Angewandte Makromolekulare Chemie 22 (1): 133–157. doi:10.1002/apmc.1972.050220107.
- ↑ Demartino, R. N. (1 August 1984). "Comfort Properties of Polybenzimidazole Fiber". Textile Research Journal 54 (8): 516–521. doi:10.1177/004051758405400803.
- ↑ Chung, Tai-Shung (1 May 1997). "A Critical Review of Polybenzimidazoles". Polymer Reviews 37 (2): 277–301. doi:10.1080/15321799708018367.
- ↑ Kricheldorf, edited by Hans R. (1992). Handbook of polymer synthesis. (dernière ed.). New York: Marcel Dekker. ISBN 0-8247-8514-2.
- ↑ Kricheldorf, edited by Hans R. (1992). Handbook of polymer synthesis. (dernière ed.). New York: Marcel Dekker. ISBN 0-8247-8514-2.
- ↑ Belohlav, Leo R. (10 December 1974). "Polybenzimidazole". Angewandte Makromolekulare Chemie 40 (1): 465–483. doi:10.1002/apmc.1974.050400122.
- ↑ Kirshenbaum, edited by Raymond B. Seymour, Gerald S. (1987). High Performance Polymers: Their Origin and Development Proceedings of the Symposium on the History of High Performance Polymers at the American Chemical Society Meeting held in New York, April 15-18, 1986. Dordrecht: Springer Netherlands. ISBN 978-94-011-7075-8.
- ↑ Sandor, R.B. (1990). "PBI (Polybenzimidazole): Synthesis, Properties and Applications". High Performance Polymers 2 (1): 25–37. doi:10.1177/152483999000200103 (inactive 2015-02-01).
- ↑ Mager, David. "EVALUATING THE RESULTS OF A MODIFIED BUNKER GEAR POLICY" (PDF). Retrieved 9 March 2014.
- ↑ Li, Qingfeng; Jensen, Jens Oluf; Savinell, Robert F.; Bjerrum, Niels J. (May 2009). "High temperature proton exchange membranes based on polybenzimidazoles for fuel cells". Progress in Polymer Science 34 (5): 449–477. doi:10.1016/j.progpolymsci.2008.12.003.
- ↑ Kumbharkar, S.C.; Li, K. (October 2012). "Structurally modified polybenzimidazole hollow fibre membranes with enhanced gas permeation properties". Journal of Membrane Science. 415-416: 793–800. doi:10.1016/j.memsci.2012.05.071.
- ↑ Li, Qingfeng; Jensen, Jens Oluf; Savinell, Robert F.; Bjerrum, Niels J. (May 2009). "High temperature proton exchange membranes based on polybenzimidazoles for fuel cells". Progress in Polymer Science 34 (5): 449–477. doi:10.1016/j.progpolymsci.2008.12.003.
- ↑ Kerres, Jochen A.; Xing, Danmin; Schönberger, Frank (15 August 2006). "Comparative investigation of novel PBI blend ionomer membranes from nonfluorinated and partially fluorinated poly arylene ethers". Journal of Polymer Science Part B: Polymer Physics 44 (16): 2311–2326. Bibcode:2006JPoSB..44.2311K. doi:10.1002/polb.20862.
- ↑ Ward, B.C (1987). "32nd SAMPE Int. Symp.," (32): 853.
- ↑ Sandor, R.B. (1990). "PBI (Polybenzimidazole): Synthesis". High Performance Polymers 2 (1): 25–37. doi:10.1177/152483999000200103 (inactive 2015-02-01).
- ↑ Sandor, R.B. (1990). "PBI (Polybenzimidazole): Synthesis, Properties and Applications". High Performance Polymers 2 (1): 25–37. doi:10.1177/152483999000200103 (inactive 2015-02-01).
- ↑ Samms, S. R. (1996). "Thermal Stability of Proton Conducting Acid Doped Polybenzimidazole in Simulated Fuel Cell Environments". Journal of the Electrochemical Society 143 (4): 1225. doi:10.1149/1.1836621.
- ↑ Wainright,, J.S.; Wang, J.-T., Weng, D., Savinell, R.F., Litt, M (July 1995). "Acid-doped polybenzimidazoles: A new polymer electrolyte". Journal of the Electrochemical Society 142 (7): L121–L123. doi:10.1149/1.2044337.
- ↑ Samms, S. R. (1996). "Thermal Stability of Proton Conducting Acid Doped Polybenzimidazole in Simulated Fuel Cell Environments". Journal of the Electrochemical Society 143 (4): 1225. doi:10.1149/1.1836621.
- ↑ Zhao, edited by T.S. (2009). Micro fuel cells : principles and applications. Burlington, MA: Academic Press. ISBN 9780123747136.
- ↑ Zhao, edited by T.S. (2009). Micro fuel cells : principles and applications. Burlington, MA: Academic Press. ISBN 9780123747136.
- ↑ Buckley, A (1988). Encyclopedia of Polymer Science And Engineering,. New York: John Wiley & Sons.
- ↑ Coffin, D.R.; Serad, G.A.; Hicks, H.L.; Montgomery, R.T. (1 July 1982). "Properties and Applications of Celanese PBI--Polybenzimidazole Fiber". Textile Research Journal 52 (7): 466–472. doi:10.1177/004051758205200706.
- ↑ Celanese. "PBI in High Temperature Protective Gloves" (PDF). Retrieved 9 March 2014.
- ↑ Hearle, ed. by J.W.S. (2004). High-performance fibres (Repr. ed.). Boca Raton, Fla. [u.a.]: CRC Press. ISBN 1855735393.
Appendix of properties
PBI fiber characteristics
The chemical formula of poly[2,2’-(m-phenylen)-5,5’ bibenzimidazol] (PBI) is believed to be: ([NH-C=CH-C=CH-CH=C-N=C-]2-[C=CH-C=CH-CH=CH-])n OR (C20N4H12)n of Molar mass 308.336 ± 0.018 g/mol.
Chemical resistance
| Chemical Resistance | Grade |
|---|---|
| Acids - concentrated | Poor |
| Acids - dilute | Fair-Poor |
| Alcohols | Good |
| Alkalis | Good-Poor |
| Aromatic hydrocarbons | Good |
| Greases and Oils | Good |
| Halogenated Hydrocarbons | Good |
| Ketones | Good |
It is dyeable to dark shades with basic dyes following caustic pretreatment and resistant to most chemicals.
Electrical properties
| Electrical | Properties |
|---|---|
| Dielectric constant @ 1 MHz | 3.2 |
| Dielectric strength | 21 kV·mm−1 |
| Volume resistivity | 8x1014 Ω·cm |
Features low electrical conductivity and low static electricity buildup.
Mechanical properties
| Mechanical | Properties |
|---|---|
| Coefficient of friction | 0.19-0.27 |
| Compressive modulus | 6.2 GPa |
| Compressive strength | 400 MPa |
| Elongation at break | 3% |
| Hardness - Rockwell | K115 |
| Izod impact strength | 590 J·m−1 unnotched |
| Poisson's ratio | 0.34 |
| Tensile modulus | 5.9 GPa |
| Tensile strength | 160 MPa |
Features abrasion resistance.
Physical Properties
| Physical | Properties |
|---|---|
| Char Yield (under pyrolysis) | High |
| Density | 1.3 g/cm3 |
| Flammability | Does not burn |
| Limiting oxygen index | 58% |
| Radiation resistance | Good |
| Water absorption - over 24 hours | 0.4% |
Additional features: will not ignite or smolder (burn slowly without flame), mildew- and age-resistant, resistant to sparks and welding spatter.
Thermal Properties
| Thermal | Properties | Grade |
|---|---|---|
| Coefficient of thermal expansion | 23×10−6·K−1 | Low |
| Heat-deflection temperature - 0.45 MPa | 435 °C (815 °F) | High |
| Thermal conductivity @ 23 °C (73 °F) | 0.41 W·m−1·K−1 | Low |
| Upper working temperature | 260–400 °C (500–752 °F) | High |
Other features: continuous temperature: 540 °C (1,004 °F), does not melt but degrades around the temperature: 760 °C (1,400 °F) under pyrolysis, retains fiber integrity and suppleness up to 540 °C (1,004 °F).
External links
- Polybenzimidazole (PBI) - Material Information
- Summary of Polybenzimidazole
- PBI Polymer Performance Study