Polyamide
A polyamide is a macromolecule with repeating units linked by amide bonds.[1] Polyamides occur both naturally and artificially. Examples of naturally occurring polyamides are proteins, such as wool and silk. Artificially made polyamides can be made through step-growth polymerization or solid-phase synthesis yielding materials such as nylons, aramids, and sodium poly(aspartate). Synthetic polyamides are commonly used in textiles, automotive applications, carpets and sportswear due to their high durability and strength. The transportation industry is the major consumer, accounting for 35% of polyamide (PA) consumption.[2]
Classification
Polymers of amino acids are known are polypeptides or proteins.
According to the composition of their main chain, synthetic polyamides are classified as follows:
| Polyamide family | Main chain | Examples of polyamides | Examples of commercial products |
|---|---|---|---|
| Aliphatic polyamides | Aliphatic | PA 6 and PA 66 | Zytel from DuPont, Technyl from Solvay, Rilsan and Rilsamid from Arkema, Radipol from Radici Group |
| Polyphthalamides | Semi-aromatic | PA 6T = hexamethylenediamine + terephthalic acid | Trogamid T from Evonik Industries, Amodel from Solvay |
| Aramides = aromatic polyamides | Aromatic | Paraphenylenediamine + terephthalic acid | Kevlar and Nomex from DuPont, Teijinconex, Twaron and Technora from Teijin, Kermel from Kermel. |
All polyamides are made by the formation of an amide function to link two molecules of monomer together. The monomers can be amides themselves (usually in the form of a cyclic lactam such as Caprolactam), α,ω-amino acids or a stoichiometric mixture of a diamine and a diacid. Both these kinds of precursors give a homopolymer. Polyamides are easily copolymerized, and thus many mixtures of monomers are possible which can in turn lead to many copolymers. Additionally many nylon polymers are miscible with one another allowing the creation of blends.
Polymerization chemistry
In living organisms, amino acids are condensed with one another by an enzyme to form amide groups (known as Peptides). The resulting polymers are known as polypeptides or proteins.
For the industrial production of synthetic polyamides with at least one aliphatic monomer ("nylon polymers"), the amide link is produced from the condensation reaction of an amino group and a carboxylic acid group, water is eliminated. For fully aromatic polyamides or 'aramids' e.g.Kevlar , the acid chloride is used as a monomer. The polymerization reaction with the amine group eliminates hydrogen chloride. The acid chloride route can be used as a laboratory synthesis to avoid heating and obtain an almost instantaneous reaction. [3]
The amino group and the carboxylic acid group can be on the same monomer, or the polymer can be constituted of two different bifunctional monomers, one with two amino groups, the other with two carboxylic acid or acid chloride groups.
Amino acids can be taken as examples of single monomer (if the difference between R groups is ignored) reacting with identical molecules to form a polyamide:
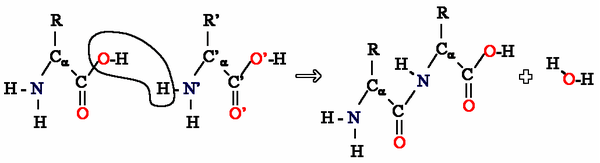
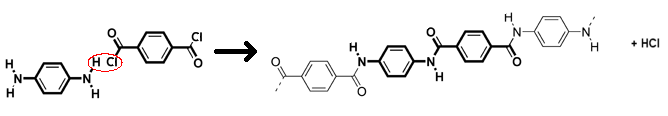
Aramid (pictured below) is made from two different monomers which continuously alternate to form the polymer and is an aromatic polyamide:
See also
References
- ↑ Palmer, R. J. 2001. Polyamides, Plastics. Encyclopedia Of Polymer Science and Technology. doi:10.1002/0471440264.pst251
- ↑ Market Study Engineering Plastics, Ceresana, Sep 2013
- ↑ "Making nylon: The "nylon rope trick"". Royal Society of Chemistry. Retrieved 19 April 2015.
Bibliography
Kohan, Melvin I. (1995). Nylon Plastics Handbook. Hanser/Gardner Publications. ISBN 9781569901892
|