Pilocarpine
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
|
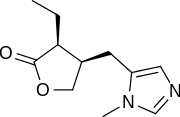
(3S,4R)-3-Ethyl-4-((1-methyl-1H-imidazol-5-yl)methyl)dihydrofuran-2(3H)-one | |
| Clinical data | |
| Trade names | Salagen |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a608039 |
| Pregnancy category | |
| Legal status | |
| Routes of administration | topical (eye drops), per os |
| Pharmacokinetic data | |
| Biological half-life | 0.76 hours (5 mg), 1.35 hours (10 mg)[1] |
| Excretion | urine |
| Identifiers | |
| CAS Number |
92-13-7 54-71-7 (hydrochloride) |
| ATC code | N07AX01 S01EB01 |
| PubChem | CID 5910 |
| IUPHAR/BPS | 305 |
| DrugBank |
DB01085 |
| ChemSpider |
5699 |
| UNII |
01MI4Q9DI3 |
| KEGG |
D00525 |
| ChEBI |
CHEBI:8207 |
| ChEMBL |
CHEMBL550 |
| Chemical data | |
| Formula | C11H16N2O2 |
| Molar mass | 208.257 g/mol |
| |
| |
| (verify) | |
Pilocarpine is a drug used to treat dry mouth and glaucoma. It is a parasympathomimetic alkaloid obtained from the leaves of tropical South American shrubs from the genus Pilocarpus. It is a non-selective muscarinic receptor agonist[2] in the parasympathetic nervous system, which acts therapeutically at the muscarinic acetylcholine receptor M3 due to its topical application,[3] e.g., in glaucoma and xerostomia.
It is on the World Health Organization's List of Essential Medicines, a list of the most important medication needed in a basic health system.[4]
Medical uses
Pilocarpine stimulates the secretion of large amounts of saliva and sweat.[5] It is used to treat dry mouth (xerostomia), particularly in Sjögren's syndrome, but also as a side effect of radiation therapy for head and neck cancer.
It has also been used in the treatment of chronic open-angle glaucoma and acute angle-closure glaucoma for over 100 years.[6] It acts on a subtype of muscarinic receptor (M3) found on the iris sphincter muscle, causing the muscle to contract -resulting in pupil constriction (miosis). Pilocarpine also acts on the ciliary muscle and causes it to contract. When the ciliary muscle contracts, it opens the trabecular meshwork through increased tension on the scleral spur. This action facilitates the rate that aqueous humor leaves the eye to decrease intraocular pressure.
In ophthalmology, pilocarpine is also used to reduce the possibility of glare at night from lights when the patient has undergone implantation of phakic intraocular lenses; the use of pilocarpine would reduce the size of the pupils, relieving these symptoms. The most common concentration for this use is pilocarpine 1%, the weakest concentration.
Pilocarpine is used to stimulate sweat glands in a sweat test to measure the concentration of chloride and sodium that is excreted in sweat. It is used to diagnose cystic fibrosis.
Adverse effects
Use of pilocarpine may result in a range of adverse effects, most of them related to its non-selective action as a muscarinic receptor agonist. Pilocarpine has been known to cause excessive salivation, sweating, bronchial mucus secretion, bronchospasm, bradycardia, vasodilation, and diarrhea. Eye drops can result in brow ache and chronic use in miosis.
Systemic injection of pilocarpine can compromise the blood brain barrier allowing pilocarpine to gain access to the brain which can lead to chronic epilepsy. Epilepsy induced by injected pilocarpine has been used to develop animal models in rodents in order to study human epilepsy.
"Scoline apnea" is a serious complication. In that condition, patient is unable to resume normal respiration after termination of general anesthesia.
Preparation
Plants in the genus Pilocarpus are the only known sources of pilocarpine, and commercial production is derived entirely from the leaves of Pilocarpus microphyllus (Maranham Jaborandi). This genus grows only in South America, and Pilocarpus microphyllus is native to several states in northern Brazil.[7]
Pilocarpine is extracted from the powdered leaf material in a multi-step process. First the material is treated with ethanol acidified with hydrochloric acid, and the solvents removed under reduced pressure. The resultant aqueous residue is neutralized with ammonia and put aside until the resin has completely settled. It is then filtered and concentrated by sugar solution to a small volume, made alkaline with ammonia, and finally extracted with chloroform. The solvent is removed under reduced pressure.
Scientific
Pilocarpine is used to induce chronic epilepsy in rodents, commonly rats, as a means to study the disorder's physiology and to examine different treatments.[8] Smaller doses may be used to induce salivation in order to collect samples of saliva, for instance, to obtain information about IgA antibodies.
Veterinary
Pilocarpine is given in moderate doses (about 2 mg) to induce emesis in cats who have ingested foreign plants, foods, or drugs. One feline trial determined it was effective, even though the usual choice of emetic is xylazine.
Trade names
Pilocarpine is available under several trade names such as: Diocarpine (Dioptic), Isopto Carpine (Alcon), Miocarpine (CIBA Vision), Ocusert Pilo-20 and -40 (Alza), Pilopine HS (Alcon), Salagen (MGI Pharma), Scheinpharm Pilocarpine (Schein Pharmaceutical), and Timpilo (Merck Frosst).
See also
- Cevimeline—a similar parasympathomimetic medication for dry mouth (xerostomia)
- Bethanechol—a similar muscarinic parasympathomimetic with longer lasting effect
References
- ↑ Mervyn Gornitsky, George Shenouda, Khalil Sultanem, Howard Katz, Michael Hier, Martin Black, Ana M Velly (2004). "Double-blind randomized, placebo-controlled study of pilocarpine to salvage salivary gland function during radiotherapy of patients with head and neck cancer". Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology 98 (1): 45–52.
- ↑ Spalding et al. 2002.
- ↑ Pharmacology, (Rang, Dale, Ritter & Moore, ISBN 0-443-07145-4, 5th ed., Churchill Livingstone 2003) p. 144.
- ↑ "WHO Model List of Essential Medicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- ↑ "Pilocarpine".
- ↑ Rosin, A. (1991). "Pilocarpine. A miotic of choice in the treatment of glaucoma has passed 110 years of use". Oftalmologia (Romania) 35 (1): 53–55. PMID 1811739.
- ↑ De Abreu, Ilka Nacif; Sawaya, Alexandra Cristine H. F.; Eberlin, Marcos Nogueira; Mazzafera, Paulo (November–December 2005). "Production of Pilocarpine in Callus of Jaborandi (Pilocarpus microphyllus Stapf)". In Vitro Cellular & Developmental Biology. Plant (Society for In Vitro Biology) 41 (6): 806–811.
- ↑ Morimoto, K; Fahnestock, M; Racine, RJ (May 2004). "Kindling and status epilepticus models of epilepsy: rewiring the brain.". Progress in neurobiology 73 (1): 1–60. doi:10.1016/j.pneurobio.2004.03.009. PMID 15193778.
Further reading
- The Italian Journal of Neurological Sciences Volume 16, Numbers 1-2, 33-37, doi:10.1007/BF02229072
- Pathophysiology of Status Epilepticus Induced by Pilocarpine, central nervous system agents in medical chemistry, 2007, vol. 7, no. 1
External links
| ||||||||||||||||||||||||||||||||||||