Piceid
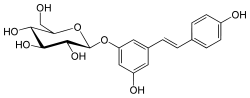 Trans-piceid | |
| Names | |
|---|---|
| IUPAC names
2-[3-Hydroxy-5-[(E)- 2-(4-hydroxyphenyl)ethenyl]phenoxy]- 6-(hydroxymethyl)oxane- 3,4,5-triol | |
| Other names
Polydatin Resveratrol 3-beta-mono-D-glucoside Cis-piceid trans-piceid 3,5,4'-trihydroxystilbene-3-O-β-D-glucopyranoside | |
| Identifiers | |
| 27208-80-6 | |
| ChEMBL | ChEMBL142652 |
| ChemSpider | 4445034 |
| Jmol interactive 3D | Image |
| PubChem | 5281718 |
| UNII | XM261C37CQ |
| |
| |
| Properties | |
| C20H22O8 | |
| Molar mass | 390.384 g/mol |
| Appearance | white powder |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Piceid is a stilbenoid glucoside and is a major resveratrol derivative in grape juices.[1] It can be found in the bark of Picea sitchensis.[2] It can also be isolated from Polygonum cuspidatum,[3] the Japanese knotweed (syn. Fallopia japonica).
Resveratrol can be produced from piceid fermented by Aspergillus oryzae.[3] This latter species produces a piceid-b-D-glucosidase.[4]
Trans-piceid is the glucoside formed with trans-resveratrol, while cis-piceid is formed with cis-resveratrol.
Trans-resveratrol-3-O-glucuronide is one of the two metabolites of trans-piceid in rat.[5]
Resveratrol glucoside from transgenic alfalfa has been used for the prevention of aberrant crypt foci in mice.[6]
See also
- Resveratroloside (3,5,4'-trihydroxystilbene-4'-O-β-D-glucopyranoside)
References
- ↑ Romero-Pérez, A. I.; Ibern-Gómez, M.; Lamuela-Raventós, R. M.; De La Torre-Boronat, M. C. (1999). "Piceid, the Major Resveratrol Derivative in Grape Juices". Journal of Agricultural and Food Chemistry 47 (4): 1533–1536. doi:10.1021/jf981024g. PMID 10564012.
- ↑ Aritomi, M.; Donnelly, D. M. X. (1976). "Stilbene glucosides in the bark of Picea sitchensis". Phytochemistry 15 (12): 2006. doi:10.1016/S0031-9422(00)88881-0.
- 1 2 Wang, H.; Liu, L.; Guo, Y. -X.; Dong, Y. -S.; Zhang, D. -J.; Xiu, Z. -L. (2007). "Biotransformation of piceid in Polygonum cuspidatum to resveratrol by Aspergillus oryzae". Applied Microbiology and Biotechnology 75 (4): 763–768. doi:10.1007/s00253-007-0874-3. PMID 17333175.
- ↑ Purification and characterization of piceid-b-D-glucosidase from Aspergillus oryzae. Chunzhi Zhang, Dai Li, Hongshan Yu, Bo Zhang and Fengxie Jin, Process Biochemistry, 2007, 42, pages 83–88, doi:10.1016/j.procbio.2006.07.019
- ↑ Zhou, M.; Chen, X.; Zhong, D. (2007). "Simultaneous determination of trans-resveratrol-3-O-glucoside and its two metabolites in rat plasma using liquid chromatography with ultraviolet detection". Journal of Chromatography B 854: 219. doi:10.1016/j.jchromb.2007.04.025.
- ↑ RKineman, B. D.; Brummer, E. C.; Paiva, N. L.; Birt, D. F. (2010). "Resveratrol from Transgenic Alfalfa for Prevention of Aberrant Crypt Foci in Mice". Nutrition and Cancer 62 (3): 351–361. doi:10.1080/01635580903407213. PMID 20358473.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
This article is issued from Wikipedia - version of the Monday, November 30, 2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.