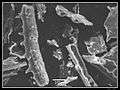
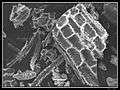
Phytolith
Phytoliths (from Greek, "plant stone") are rigid, microscopic structures made of silica, found in some plant tissues and persisting after the decay of the plant. These plants take up silica from the soil, whereupon it is deposited within different intracellular and extracellular structures of the plant. Phytoliths come in varying shapes and sizes. Although some use "phytolith" to refer to all mineral secretions by plants, it more commonly refers to siliceous plant remains. In contrast, mineralized calcium secretions in cacti are composed of calcium oxalates.[1]
Functions
There is still debate in the scientific community as to why plants form phytoliths, and whether silica should be considered an essential nutrient for plants. Studies that have grown plants in silica-free environments have typically found that plants lacking silica in the environment do not grow as well. For example, the stems of certain plants will collapse when grown in soil lacking silica. In many cases, phytoliths appear to lend structure and support to the plant, much like the spicules in sponges and leather corals. Phytoliths may also provide plants with protection. These rigid silica structures help to make plants more difficult to consume and digest, lending the plant's tissues a grainy or prickly texture.[2] Phytoliths also appear to provide physiologic benefits. Experimental studies have shown that the silicon dioxide in phytoliths may help to alleviate the damaging effects of toxic heavy metals, such as aluminum. Finally, calcium oxalates serve as a reserve of carbon dioxide. Cacti use these as a reserve for photosynthesis during the day when they close their pores to avoid water loss; baobabs use this property to make their trunks more flame-resistant.
History of phytolith research
According to Dolores Piperno, an expert in the field of phytolith analysis, there have been four important stages of phytolith research throughout history.[1][3]
- Discovery and exploratory stage (1835–1895): The first report on phytoliths was published by a German botanist named Struve in 1835. During this time another German scientist named Christian Gottfried Ehrenberg was one of the leaders in the field of phytolith analysis. He developed the first classification system for phytoliths, and analyzed soil samples that were sent to him from all around the world. Most notably, Ehrenberg recorded phytoliths in samples he received from the famous naturalist, Charles Darwin, who had collected the dust from the sails of his ship, the HMS Beagle, off the coast of the Cape Verde Islands.
- Botanical phase of research (1895–1936): Phytolith structures in plants gained wide recognition and attention throughout Europe. Research on production, taxonomy and morphology exploded. Detailed notes and drawings on plant families that produce silica structures and morphology within families were published.
- Period of ecological research (1955–1975): First applications of phytolith analysis to paleoecological work, mostly in Australia, the United States, the United Kingdom, and Russia. Classification systems for differentiation within plant families became popular.
- Modern period of archaeological and paleoenvironmental research (1978–present): Archaeobotanists working in the Americas first consider and analyze phytolith assemblages in order to track prehistoric plant use and domestication. Also for the first time, phytolith data from pottery are used to track history of clay procurement and pottery manufacture. Around the same time, phytolith data are also used as a means of vegetation reconstruction among paleoecologists. A much larger reference collection on phytolith morphology within varying plant families is assembled.
Development in plants
First, soluble silica, also called monosilicic acid, is taken up from the soil when plant roots absorb groundwater. From there, it is carried to other plant organs by the xylem. By an unknown mechanism, which appears to be linked to genetics and metabolism, some of the silica is then laid down in the plant as silicon dioxide. This biological mechanism does not appear to be limited to specific plant structures, as some plants have been found with silica in their reproductive and sub-surface organs.[1]
Chemical and physical characteristics
Phytoliths are composed mainly of noncrystalline silicon dioxide, and about 4% to 9% of their mass is water. Carbon, nitrogen, and other major nutrient elements comprise less than 5%, and commonly less than 1%, of phytolith material by mass. These elements are present in the living cells in which the silica concretions form, so traces are retained in the phytoliths. Such immobilised elements, in particular carbon, are valuable in that they permit radiometric dating in reconstructing past vegetation patterns. The silica in phytoliths has a refractive index ranging from 1.41 to 1.47, and a specific gravity from 1.5 to 2.3. Phytoliths may be colorless, light brown, or opaque; most are transparent. Phytoliths exist in various three-dimensional shapes, some of which are specific to plant families, genera or species.
Single cell and conjoined phytoliths
Phytoliths may form within single cells, or multiple cells within a plant to form 'conjoined' or multi-cell phytoliths, which are three-dimensional replicas of sections of plant tissue. Conjoined phytoliths occur when conditions are particularly favourable for phytolith formation, such as on a silica rich substrate with high water availability[4]
Patterns of phytolith production
Because identification of phytoliths is based on morphology, it is important to note taxonomical differences in phytolith production.[1]
Families with high phytolith production; family and genus-specific phytolith morphology is common:
- Acanthaceae, Aceraceae, Annonaceae, Arecaceae, Asteraceae, Boraginaceae, Bromeliaceae, Burseraceae, Chrysobalanaceae, Commelinaceae, Costaceae, Cucurbitaceae, Cyatheaceae, Cyperaceae, Dilleniaceae, Equisetaceae, Heliconiaceae, Hymenophyllaceae, Magnoliaceae, Marantaceae, Moraceae, Musaceae, Orchidaceae, Poaceae, Podostemaceae, Selaginellaceae, Ulmaceae, Urticaceae, Zingiberaceae
Families where phytolith production may not be high; family and genus-specific phytolith morphology is common:
- Capparaceae, Cupressaceae, Dipterocarpaceae, Euphorbiaceae, Fagaceae, Flacourtiaceae, Flagellariaceae, Joinvilleaceae, Pinaceae, Polypodiaceae, Restionaceae, Taxaceae, Taxodiaceae
Families where phytolith production is common; family and genus-specific phytolith morphology is uncommon:
- Aristolochiaceae, Chloranthaceae, Combretaceae, Hernandiaceae, Loranthaceae, Menispermaceae, Piperaceae, Sapotaceae, Verbenaceae
Families where phytolith productions varies; family and genus-specific phytolith morphology is uncommon:
Families where phytolith production is rare or not observed:
- Agavaceae, Alismataceae, Amaranthaceae, Amaryllidaceae, Apiaceae, Apocynaceae, Araceae, Araliaceae, Araucariaceae, Asclepiadaceae, Bignoniaceae, Bixaceae, Bombacaeae, Burmanniaceae, Cactaceae, Campanulaceae, Caricaceae, Cartonemataceae, Chenopodiaceae, Convolvulaceae, Cycadaceae, Cyclanthaceae, Dioscoreaceae, Ericaceae, Eriocaulaceae, Gnetaceae, Guttiferae, Hydrocharitaceae, Iridaceae, Juglandaceae, Juncaceae, Labiatae, Lacistemnaceae, Lauraceae, Lecythidaceae, Lentibulariaceae, Liliaceae, Loganiaceae, Malphigiaceae, Mayacaceae, Melastomataceae, Meliaceae, Myristicaceae, Myrtaceae, Myrsinaceae, Nymphaeaceae, Olacaceae, Oxalidaceae, Pedaliaceae, Podocarpaceae, Polygonaceae, Pontederiaceae, Potamogetonaceae, Primulaceae, Proteaceae, Ranunculaceae, Rhamnaceae, Rosaceae, Rubiaceae, Rutaceae, Salicaceae, Sapindaceae, Saxifragaceae, Smilacaceae, Solanaceae, Theaceae, Tiliaceae, Trioridaceae, Typhaceae, Vitaceae, Violaceae, Winteraceae, Xyridaceae, Zygophyllaceae
Archaeology
Phytoliths are very robust, and are useful in archaeology because they can help to reconstruct the plants present at a site when the rest of the plant parts have been burned up or dissolved. Because they are made of the inorganic substances silica or calcium oxalate, phytoliths don't decay with the rest of the plant and can survive in conditions that would destroy organic residues. Phytoliths can provide evidence of both economically important plants and those that are indicative of the environment at a particular time period.
Phytoliths may be extracted from residue on many sources: dental calculus (buildup on teeth); food preparation tools like rocks, grinders, and scrapers; cooking or storage containers; ritual offerings; and garden areas.
Sampling strategies
- Cultural contexts: The most important consideration when designing a sampling strategy for a cultural context is to fit the sampling design to the research objectives. For example, if the objective of the study is to identify activity areas, it may be ideal to sample using a grid system. If the objective is to identify foodstuffs, it may be more beneficial to focus on areas where food processing and consumption took place. It is always beneficial to sample ubiquitously throughout the site, because it is always possible to select a smaller portion of the samples for analysis from a larger collection. Samples should be collected and labeled in individual plastic bags. It is not necessary to freeze the samples, or treat them in any special way because silica is not subject to decay by microorganisms.[5]
- Natural contexts: Sampling a natural context, typically for the purpose of environmental reconstruction, should be done in a context that is free of disturbances. Human activity can alter the makeup of samples of local vegetation, so sites with evidence of human occupation should be avoided. Bottom deposits of lakes are usually a good context for phytolith samples, because wind often will carry phytoliths from the topsoil and deposit them on water, where they will sink to the bottom, very similar to pollen. It is also possible and desirable to take vertical samples of phytolith data, as it can be a good indicator of changing frequencies of taxa over time.[5]
- Modern surfaces: Sampling modern surfaces for use with archeobotanical data may be used to create a reference collection, if the taxa being sampled are known. It may also serve to "detect downward movement of phytoliths into archaeological strata".[5] Taking point samples for modern contexts is ideal.
Laboratory analysis

The first step in the laboratory analysis of phytolith samples is processing, in order to extract the phytoliths from the soil. Phytoliths can be extracted from soil samples in two ways: chemically or by ashing. After processing, microscopy is used to identify the phytoliths. Optical microscopes with magnifications of 200-400x are typically used to screen phytoliths. Scanning electron microscopy may also allow for a more detailed study of phytoliths.[5]
Contribution to archaeobotanical knowledge
- Phytolith analysis is particularly useful in tropical regions, where other types of plant remains are typically not well preserved.
- Phytolith analysis has been used to retrace the domestication and ancestral lineage of various plants. For example, research tracing modern lineages of maize in South America and the American Southwest using phytolith remains on ceramics and pottery has proven to be enlightening. Recent genetic data suggests that the oldest ancestor of Zea mays is teosinte, a wild grass found in southwest Mexico. The Zea mays lineage split off from this grass about six to seven thousand years ago. Phytolith analyses from Bolivia suggest that several varieties of maize were present in the Lake Titicaca region of Bolivia almost 1000 years before the Tiwanaku expansion, when it was previously thought to have been introduced in the region. This case is not isolated. Around the same time, certain varieties of maize could be found with ubiquity across part of South America, suggesting a highly frequented and established trade route existed. Phytolith data from the southeastern United States suggest that two different lineages of maize were introduced from two different sources. Research that hopes to discover more specific information about the spread of maize throughout the southeastern United States is currently under way.[6]
- To date, phytolith analyses have also been popular for studies of rice. Because the morphology of rice phytoliths has been significantly documented, studies concerning the domestication of rice, as well as crop processing models using phytolith analyses, are insightful. In one study, phytolith analysis was used to complement macro-remains sampling in order to infer concentrations of plant parts and predict crop processing stages.[7]
- Phytolith analysis has been useful in identifying early agriculture in South East Asia during the Early Holocene.[8][9]
Problems with phytolith analysis of remains
- Multiplicity- different parts of a single plant may produce different phytoliths.
- Redundancy- different plants can produce the same kind of phytolith.[10]
It is suggested that using phytolith data from food residues (on ceramics, usually) can decrease the bias from both of these problems, because phytolith analysis is more likely to represent crop products and identification of phytoliths can be made with more confidence. Also, food residues do not usually accumulate extraneous deposits. In other words, the samples are more likely to represent a primary context.[6]
Palaeontology
Phytoliths are abundant in the fossil record,[11] and have been reported from the Late Devonian onwards.[11] They can be used to identify palaeoenvironments and track vegetational change.[11]
Occasionally, paleontologists find and identify phytoliths associated with extinct plant-eating animals (i.e. herbivores). Findings such as these reveal useful information about the diet of these extinct animals, and also shed light on the evolutionary history of many different types of plants. Paleontologists in India have recently identified grass phytoliths in dinosaur dung (coprolites), strongly suggesting that the evolution of grasses began earlier than previously thought.[12]
Japanese and Korean archaeologists refer to grass and crop plant phytoliths as 'plant opal' in archaeological literature.
Phytolith Gallery
Carbon sequestration
Research, particularly since 2005 has shown that carbon in phytoliths can be resistant to decomposition for millennia and can accumulate in soils.[13] While researchers had previously known that phytoliths could persist in some soils for thousands of years [14] and that there was carbon occluded within phytoliths that could be used for radiocarbon dating,[15] research into the capacity of phytoliths as a method of storing carbon in soils was pioneered by Parr and Sullivan [16] suggested that there was a real opportunity to sequester carbon securely in soils for the long term, in the form of carbon inclusions in durable silica phytoliths. Subsequent research has shown the effectiveness of phytoliths as a process to sequester carbon using a range of agricultural crops and other economically important plants While carbon sequestration is a potentially important way to limit atmospheric greenhouse gas concentrations in the long term, the use of phytoliths to achieve this must be balanced against other uses that might be made of the same biomass carbon (or land for producing biomass) to reduce GHG emissions by other means including, for example, the production of bioenergy to offset fossil fuel emissions. If enhanced phytolith production results in a reduced availability of biomass for other GHG mitigation strategies, its effectiveness for lowering net GHG emissions may be reduced or negated.
See also
- Druse (botany) crystals of calcium oxalate, silicates, or carbonates present in plants
- Raphide elongate calcium oxalate crystals in plants
References
- 1 2 3 4 Piperno, Dolores R. (2006). Phytoliths: A Comprehensive Guide for Archaeologists and Paleoecologists. AltaMira Press ISBN 0759103852.
- ↑ Hunt JW, Dean AP, Webster RE, Johnson GN, Ennos AR (2008) A novel mechanism by which silica defends grasses against herbivory. Ann Bot 102 (4):653-6. doi:10.1093/aob/mcn130 PMID 18697757
- ↑ Phytoliths Gallery. Smithsonian National Museum of Natural History.
- ↑ Jenkins, Emma (2009). "Phytolith taphonomy: a comparison of dry ashing and acid extraction on the breakdown of conjoined phytoliths formed in Triticum durum". Journal of Archaeological Science 36: 2402-2407. doi:10.1016/j.jas.2009.06.028.
- 1 2 3 4 Pearsall, Deborah. (2000). Paleoethnobotany: a Handbook of Procedures. Academic Press: San Diego ISBN 0125480423.
- 1 2 Lustek, Robert Karl. (2008). Setting the Archaeology of Maize on Its Ear: The Use of Phytolith Assemblages to Identify Lineages of Maize. University of Minnesota ISBN 0549717765.
- ↑ Harvey, Emma L.; Fuller, Dorian Q (2005). "Investigating crop processing using phytolith analysis: the example of rice and millets" (PDF). Journal of Archaeological Science 32 (5): 739. doi:10.1016/j.jas.2004.12.010. JSTOR 5647.
- ↑ Kealhofer L 2003. Looking into the gap: land use and the tropical forests of southern Thailand. Asian Perspectives 42(1)
- ↑ Kealhofer L 2002. Changing perceptions of risk: The development of agro-ecosystems in Southeast Asia. American Anthropologist 104(1).
- ↑ Shillito, L-M. (2013). "Grains of truth or transparent blindfolds? A review of current debates in archaeological phytolith analysis". Vegetation History and Archaeobotany 22 (1): 71–82. doi:10.1007/s00334-011-0341-z.
- 1 2 3 Carter, J.A. (1999). "Late Devonian, Permian and Triassic phytoliths from Antarctica". Micropaleontology 45 (1): 56–61. doi:10.2307/1486202. JSTOR 1486202.
- ↑ Hecht, Jeff, Fossil dung reveals dinosaurs did graze grass, New Scientist Magazine , 17 November 2005. Accessed January 2008
- ↑ Parr, J; Sullivan, L (2005). "Soil carbon sequestration in phytoliths". Soil Biology and Biochemistry 37: 117–124. doi:10.1016/j.soilbio.2004.06.013.
- ↑ Piperno, D.R., & Hans-Dieter, S. (2005). Dinosaurs Dined on Grass. Science, 310, 1126-1128.
- ↑ Wilding, L.P. (1967). Radiocarbon dating of biogenetic opal. Science, 156, 66 - 67.
- ↑ Parr, J. F. and Sullivan, L. A. (2005). "Soil carbon sequestration in Phytoliths." Soil Biology and Biochemistry. 37(1): 117-124.
- Kealhofer L 2002. Changing perceptions of risk: The development of agro-ecosystems in Southeast Asia. American Anthropologist 104(1).
Bibliography
- Thorn, V. C. 2004. An annotated bibliography of phytolith analysis and atlas of selected New Zealand subantarctic and subalphine phytoliths.
- Fabrice Colin; Jean Dominique Meunier (2001). Phytoliths. Washington, DC: Taylor & Francis. ISBN 90-5809-345-X.
- Kealhofer, L. 1998. Opal phytoliths in Southeast Asian flora.
- Jr, George V. Roberts; Susan C. Mulholland; Rapp, George Robert (1992). Phytolith systematics: emerging issues. New York: Plenum Press. ISBN 0-306-44208-6.
- Ciochon, RL; Piperno, DR; Thompson, RG (1990). "Opal phytoliths found on the teeth of the extinct ape Gigantopithecus blacki: implications for paleodietary studies.". Proceedings of the National Academy of Sciences of the United States of America 87 (20): 8120–8124. Bibcode:1990PNAS...87.8120C. doi:10.1073/pnas.87.20.8120. PMC 54904. PMID 2236026.
- Piperno, Dolores R. (1988). Phytolith analysis: an archaeological and geological perspective. Boston: Academic Press. ISBN 0-12-557175-5.
- Twiss, P. C., Suess, E., & Smith, R. M. (1969). "Morphological classification of grass phytoliths" (PDF). Proc. Soil Sci. Soc. America 33 (1): 109. doi:10.2136/sssaj1969.03615995003300010030x.
- Pearsall, Deborah M. (2004). Plants and people in ancient Ecuador: the ethnobotany of the Jama River Valley. Belmont, CA: Wadsworth/Thompson Learning. ISBN 0-534-61321-7.
- Pearsall, Deborah M. (2000). Paleoethnobotany: a handbook of procedures (2nd ed.). Boston: Academic Press. ISBN 0-12-548042-3.
- Pearsall, Deborah M.; Piperno, Dolores R. (1998). The origins of agriculture in the lowland neotropics. Boston: Academic Press. ISBN 0-12-557180-1.
- Pearsall, D (2004). "Maize in ancient Ecuador: results of residue analysis of stone tools from the Real Alto site". Journal of Archaeological Science 31 (4): 423–442. doi:10.1016/j.jas.2003.09.010.
- Darwin, C. (1846). "An account of the Fine Dust which often falls on Vessels in the Atlantic Ocean.". Quarterly Journal of the Geological Society 2: 26. doi:10.1144/GSL.JGS.1846.002.01-02.09.
- Kealhofer L 2002. Changing perceptions of risk: The development of agro-ecosystems in Southeast Asia. American Anthropologist 104(1)
External links
- What is the phytolith?
- Ecological significance of phytoliths
- Background from St. Cloud laboratory
- Association of Environmental Archaeology
- Russian Scientific Association for Phytolith Research
- Steve Archer, "About Phytoliths": http://research.history.org/Archaeological_Research/Collections/CollArchaeoBot/PhytoFAQs.cfm .
- Terry B. Ball, "Phytolith Literature Review": http://www.ou.edu/cas/botany-micro/ben/ben282.html .
- Dr. Sanjay Eksambekar's 'Phytolith Research Institute': http://www.phytolithresearch.com
- Deborah Pearsall's MU Phytolith Database, http://web.missouri.edu/~umcasphyto/index.shtml
- "What are Phytoliths?" Sandstone Archaeology Paleoethnobotany Laboratory http://www.sandstonearchaeology.com/paleoethnobotany.html