Physical constant
A physical constant is a physical quantity that is generally believed to be both universal in nature and constant in time. It can be contrasted with a mathematical constant, which is a fixed numerical value, but does not directly involve any physical measurement.
There are many physical constants in science, some of the most widely recognized being the speed of light in vacuum c, the gravitational constant G, Planck's constant h, the electric constant ε0, and the elementary charge e. Physical constants can take many dimensional forms: the speed of light signifies a maximum speed limit of the Universe and is expressed dimensionally as length divided by time; while the fine-structure constant α, which characterizes the strength of the electromagnetic interaction, is dimensionless.
Dimensional and dimensionless physical constants
Whereas the physical quantity indicated by any physical constant does not depend on the unit system used to express the quantity, the numerical values of dimensional physical constants do depend on choice of unit system. Therefore, these numerical values (such as 299,792,458 for the constant speed of light c expressed in units of meters per second) are not values that a theory of physics can be expected to predict.
Because their units cancel, ratios of like-dimensioned physical constants do not depend on unit systems in this way, so they are pure dimensionless numbers whose values any other civilization, anywhere, and at any time in the universe would predict. Additionally, all equations describing laws of physics can be expressed without dimensional physical constants via a process known as nondimensionalisation, but the dimensionless constants will remain. Thus, theoretical physicists tend to regard these dimensionless quantities as fundamental physical constants.
However, the term fundamental physical constant is also used in other ways. For example, the National Institute of Standards and Technology[1] uses the term to refer to any universal physical quantity believed to be constant, such as the speed of light c, and the gravitational constant G.
The fine-structure constant α is probably the best known dimensionless fundamental physical constant. Many attempts have been made to derive its value (currently measured at about 1/137.035999) from theory, but so far none have succeeded. The same holds for the dimensionless ratios of masses of fundamental particles (such as mp/me, approximately 1836.152672). With the development of quantum chemistry in the 20th century, however, a vast number of previously inexplicable dimensionless physical constants were successfully computed from theory. In light of that, some theoretical physicists still hope for continued progress in explaining the values of other dimensionless physical constants.
It is known that the Universe would be very different, if these constants took values significantly different from those we observe. For example, a few percent change in the value of the fine structure constant would be enough to eliminate stars like our Sun. This has prompted attempts at anthropic explanations of the values of some of the dimensionless fundamental physical constants.
How constant are the physical constants?
Beginning with Paul Dirac in 1937, some scientists have speculated that physical constants may actually decrease in proportion to the age of the Universe. Scientific experiments have not yet pinpointed any definite evidence that this is the case, although they have placed upper bounds on the maximum possible relative change per year at very small amounts (roughly 10−17 per year for the fine structure constant α and 10−11 for the gravitational constant G).
It is currently disputed[2][3] whether any changes in dimensional physical constants such as G, c, ħ, or ε0 are operationally meaningful;[4] however, a sufficient change in a dimensionless constant such as α is generally agreed to be something that would definitely be noticed. If a measurement indicated that a dimensional physical constant had changed, this would be the result or interpretation of a more fundamental dimensionless constant changing, and which would be the salient metric. From Barrow (2002):[5]
[An] important lesson we learn from the way that pure numbers like α define the World is what it really means for worlds to be different. The pure number we call the fine structure constant and denote by α is a combination of the electron charge, e, the speed of light, c, and Planck's constant, h. At first we might be tempted to think that a world in which the speed of light was slower would be a different world. But this would be a mistake. If c, h, and e were all changed so that the values they have in metric (or any other) units were different when we looked them up in our tables of physical constants, but the value of α remained the same, this new world would be observationally indistinguishable from our World. The only thing that counts in the definition of worlds are the values of the dimensionless constants of Nature. If all masses were doubled in value you cannot tell, because all the pure numbers defined by the ratios of any pair of masses are unchanged.
On December 13, 2012, physicists reported the constancy, over space and time, of a basic physical constant of nature that supports the standard model of physics. The scientists, studying methanol molecules in a distant galaxy, found the change (∆μ/μ) in the proton-to-electron mass ratio μ to be equal to "(0.0 ± 1.0) × 10−7 at redshift z = 0.89" and consistent with "a null result".[6][7]
Anthropic principle
Some physicists have explored the notion that if the dimensionless physical constants had sufficiently different values, our Universe would be so radically different that intelligent life would probably not have emerged, and that our Universe therefore seems to be fine-tuned for intelligent life. The anthropic principle states a logical truism: the fact of our existence as intelligent beings who can measure physical constants requires those constants to be such that beings like us can exist. There are a variety of interpretations of the constants' values, including that of a divine creator (the apparent fine-tuning is actual and intentional), or that ours is one universe of many in a multiverse (e.g. the Many-worlds interpretation of quantum mechanics), or even that, if information is an innate property of the universe and logically inseparable from consciousness, a universe without the capacity for conscious beings cannot exist.
Table of universal constants
Quantity |
Symbol | Value[8][9] | Relative Standard Uncertainty |
|---|---|---|---|
| speed of light in vacuum |  |
299 792 458 m·s−1 | defined |
| Newtonian constant of gravitation | 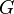 |
6.67408(31)×10−11 m3·kg−1·s−2 | 4.7 × 10−5 |
| Planck constant |  |
6.626 070 040(81) × 10−34 J·s | 1.2 × 10−8 |
| reduced Planck constant | 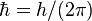 |
1.054 571 800(13) × 10−34 J·s | 1.2 × 10−8 |
Table of electromagnetic constants
Quantity |
Symbol | Value[8][10] (SI units) | Relative Standard Uncertainty |
|---|---|---|---|
| magnetic constant (vacuum permeability) |  |
4π × 10−7 N·A−2 = 1.256 637 061... × 10−6 N·A−2 | defined |
| electric constant (vacuum permittivity) | 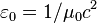 |
8.854 187 817... × 10−12 F·m−1 | defined |
| characteristic impedance of vacuum | 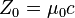 |
376.730 313 461... Ω | defined |
| Coulomb's constant | 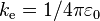 |
8.987 551 787... × 109 N·m2·C−2 | defined |
| elementary charge |  |
1.602 176 565(35) × 10−19 C | 2.2 × 10−8 |
| Bohr magneton | 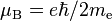 |
9.274 009 68(20) × 10−24 J·T−1 | 2.2 × 10−8 |
| conductance quantum | 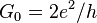 |
7.748 091 7346(25) × 10−5 S | 3.2 × 10−10 |
| inverse conductance quantum | 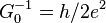 |
12 906.403 7217(42) Ω | 3.2 × 10−10 |
| Josephson constant | 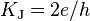 |
4.835 978 70(11) × 1014 Hz·V−1 | 2.2 × 10−8 |
| magnetic flux quantum | 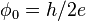 |
2.067 833 758(46) × 10−15 Wb | 2.2 × 10−8 |
| nuclear magneton | 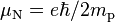 |
5.050 783 53(11) × 10−27 J·T−1 | 2.2 × 10−8 |
| von Klitzing constant | 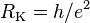 |
25 812.807 4434(84) Ω | 3.2 × 10−10 |
Table of atomic and nuclear constants
Quantity |
Symbol | Value[8][10] (SI units) | Relative Standard Uncertainty | |
|---|---|---|---|---|
| Bohr radius | 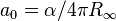 |
5.291 772 1092(17) × 10−11 m | 3.2 × 10−9 | |
| classical electron radius | 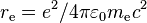 |
2.817 940 3267(27) × 10−15 m | 9.7 × 10−10 | |
| electron mass | 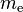 |
9.109 382 91(40) × 10−31 kg | 4.4 × 10−8 | |
| Fermi coupling constant | 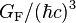 |
1.166 364(5) × 10−5 GeV−2 | 4.3 × 10−6 | |
| fine-structure constant | 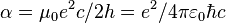 |
7.297 352 5698(24) × 10−3 | 3.2 × 10−10 | |
| Hartree energy | 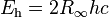 |
4.359 744 34(19) × 10−18 J | 4.4 × 10−8 | |
| proton mass | 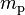 |
1.672 621 777(74) × 10−27 kg | 4.4 × 10−8 | |
| quantum of circulation | 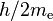 |
3.636 947 5520(24) × 10−4 m2 s−1 | 6.5 × 10−10 | |
| Rydberg constant | 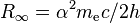 |
10 973 731.568 539(55) m−1 | 5.0 × 10−12 | |
| Thomson cross section | 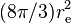 |
6.652 458 734(13) × 10−29 m2 | 1.9 × 10−9 | |
| weak mixing angle | 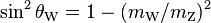 |
0.2223(21) | 9.5 × 10−3 | |
| Efimov factor | 22.7 | |||
Table of physico-chemical constants
Quantity |
Symbol | Value[8][10] (SI units) | Relative Standard Uncertainty | |
|---|---|---|---|---|
| Atomic mass constant | 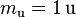 |
1.660 538 921(73) × 10−27 kg | 4.4 × 10−8 | |
| Avogadro's number | 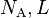 |
6.022 141 29(27) × 1023 mol−1 | 4.4 × 10−8 | |
| Boltzmann constant | 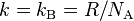 |
1.380 6488(13) × 10−23 J·K−1 | 9.1 × 10−7 | |
| Faraday constant | 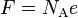 |
96 485.3365(21)C·mol−1 | 2.2 × 10−8 | |
| first radiation constant | 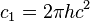 |
3.741 771 53(17) × 10−16 W·m2 | 4.4 × 10−8 | |
| for spectral radiance | 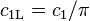 |
1.191 042 869(53) × 10−16 W·m2·sr−1 | 4.4 × 10−8 | |
| Loschmidt constant | at  =273.15 K and =273.15 K and 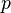 =101.325 kPa =101.325 kPa |
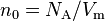 |
2.686 7805(24) × 1025 m−3 | 9.1 × 10−7 |
| gas constant | 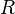 |
8.314 4621(75) J·K−1·mol−1 | 9.1 × 10−7 | |
| molar Planck constant | 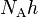 |
3.990 312 7176(28) × 10−10 J·s·mol−1 | 7.0 × 10−10 | |
| molar volume of an ideal gas | at  =273.15 K and =273.15 K and 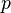 =100 kPa =100 kPa |
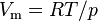 |
2.271 0953(21) × 10−2 m3·mol−1 | 9.1 × 10−7 |
at  =273.15 K and =273.15 K and 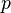 =101.325 kPa =101.325 kPa |
2.241 3968(20) × 10−2 m3·mol−1 | 9.1 × 10−7 | ||
| Sackur-Tetrode constant | at  =1 K and =1 K and 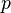 =100 kPa =100 kPa |
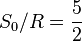 ![+ \ln\left[ (2\pi m_{\mathrm{u}} k T / h^2)^{3/2} k T / p \right]](../I/m/6c52864d043f313e34146a00ea998299.png) |
−1.151 7078(23) | 2.0 × 10−6 |
at  =1 K and =1 K and 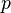 =101.325 kPa =101.325 kPa |
−1.164 8708(23) | 1.9 × 10−6 | ||
| second radiation constant | 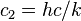 |
1.438 7770(13) × 10−2 m·K | 9.1 × 10−7 | |
| Stefan–Boltzmann constant | 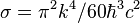 |
5.670 373(21) × 10−8 W·m−2·K−4 | 3.6 × 10−6 | |
| Wien displacement law constant | 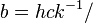 4.965 114 231... 4.965 114 231... |
2.897 7721(26) × 10−3 m·K | 9.1 × 10−7 | |
Table of adopted values
| Quantity | Symbol | Value (SI units) | Relative Standard Uncertainty | |
|---|---|---|---|---|
| conventional value of Josephson constant[11] | 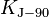 |
4.835 979 × 1014 Hz·V−1 | defined | |
| conventional value of von Klitzing constant[12] | 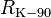 |
25 812.807 Ω | defined | |
| molar mass | constant | 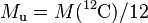 |
1 × 10−3 kg·mol−1 | defined |
| of carbon-12 | 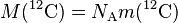 |
1.2 × 10−2 kg·mol−1 | defined | |
| standard acceleration of gravity (gee, free-fall on Earth) | 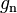 |
9.806 65 m·s−2 | defined | |
| standard atmosphere | 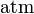 |
101 325 Pa | defined | |
Natural units
Using dimensional analysis, it is possible to combine dimensional universal physical constants to define a system of units of measurement that has no reference to any human construct. Depending on the choice and arrangement of constants used, the resulting natural units may have useful physical meaning. For example, Planck units, shown below, use c, G, ħ, ε0 and kB in such a manner to derive units relevant to unified theories such as quantum gravity.
| Base Planck units | |||
|---|---|---|---|
| Name | Dimension | Expression | Value[13] (SI units) |
| Planck length | Length (L) | 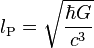 |
1.616 199(97) × 10−35 m[14] |
| Planck mass | Mass (M) | 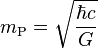 |
2.176 51(13) × 10−8 kg[15] |
| Planck time | Time (T) | 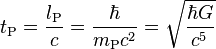 |
5.391 06(32) × 10−44 s[16] |
| Planck charge | Electric charge (Q) | 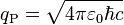 |
1.875 545 956(41) × 10−18 C[17][18][19] |
| Planck temperature | Temperature (Θ) | 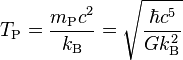 |
1.416 833(85) × 1032 K[20] |
See also
References
- ↑ 2010 Values of the Constants; NIST, 2011.
- ↑ Duff, Michael J. "Comment on time-variation of fundamental constants." High Energy Physics - Theory, 2004.
- ↑ Duff, M. J.; Okun, L. B.; Veneziano, G. "Trialogue on the number of fundamental constants." Classical Physics, 2002.
- ↑ In SI, c, and ε0 now are defined numerical values, independent of experiment, so observations now are directed elsewhere.
- ↑ Barrow, John D. (2002), The Constants of Nature; From Alpha to Omega - The Numbers that Encode the Deepest Secrets of the Universe, Pantheon Books, ISBN 0-375-42221-8
- ↑ Bagdonaite, Julija; Jansen, Paul; Henkel, Christian; Bethlem, Hendrick L.; Menten, Karl M.; Ubachs, Wim (December 13, 2012). "A Stringent Limit on a Drifting Proton-to-Electron Mass Ratio from Alcohol in the Early Universe". Science. Bibcode:2013Sci...339...46B. doi:10.1126/science.1224898. Retrieved December 14, 2012.
- ↑ Moskowitz, Clara (December 13, 2012). "Phew! Universe's Constant Has Stayed Constant". Space.com. Retrieved December 14, 2012.
- 1 2 3 4 The values are given in the so-called concise form; the number in parentheses after the mantissa is the standard uncertainty, which is the value multiplied by the relative standard uncertainty, and indicates the amount by which the least significant digits of the value are uncertain. For example, 75 is the standard uncertainty in "8.314 4621(75)", and means that the value is between 8.314 4546 and 8.314 4696.
- ↑ Mohr, Peter J.; Newell, David B.; Taylor, Barry N. "CODATA Recommended Values of the Fundamental Physical Constants: 2014". arXiv:1507.07956v1 [physics.atom-ph].
- 1 2 3 P.J. Mohr, B.N. Taylor, and D.B. Newell (2011), "The 2010 CODATA Recommended Values of the Fundamental Physical Constants" (Web Version 6.0). This database was developed by J. Baker, M. Douma, and S. Kotochigova. Available: http://physics.nist.gov/constants [Thursday, 02-Jun-2011 21:00:12 EDT]. National Institute of Standards and Technology, Gaithersburg, MD 20899.
- ↑ This is the value adopted internationally for realizing representations of the volt using the Josephson effect.
- ↑ This is the value adopted internationally for realizing representations of the ohm using the quantum Hall effect.
- ↑ Fundamental Physical Constants from NIST
- ↑ CODATA — Planck length
- ↑ CODATA — Planck mass
- ↑ CODATA — Planck time
- ↑ CODATA — electric constant
- ↑ CODATA — Planck constant over 2 pi
- ↑ CODATA — speed of light in vacuum
- ↑ CODATA — Planck temperature
- Mohr, Peter J.; Taylor, Barry N.; Newell, David B. (2008). "CODATA Recommended Values of the Fundamental Physical Constants: 2006". Rev. Mod. Phys. 80 (2): 633–730. arXiv:0801.0028. Bibcode:2008RvMP...80..633M. doi:10.1103/RevModPhys.80.633.
- Barrow, John D. (2002), The Constants of Nature; From Alpha to Omega - The Numbers that Encode the Deepest Secrets of the Universe, Pantheon Books, ISBN 0-375-42221-8.
External links
- Sixty Symbols, University of Nottingham
- IUPAC - Gold Book
|