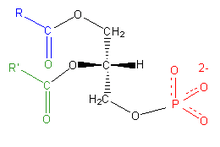
Phosphatidylcholine
Phosphatidylcholines (PC) are a class of phospholipids that incorporate choline as a headgroup. They are a major component of biological membranes and can be easily obtained from a variety of readily available sources, such as egg yolk or soybeans, from which they are mechanically or chemically extracted using hexane. They are also a member of the lecithin group of yellow-brownish fatty substances occurring in animal and plant tissues. Dipalmitoyl phosphatidylcholine (aka: lecithin) is a major component of pulmonary surfactant and is often used in the L/S ratio to calculate fetal lung maturity. While phosphatidylcholines are found in all plant and animal cells, they are absent in the membranes of most bacteria,[1] including Escherichia coli.[2] Purified phosphatidylcholine is produced commercially.
The name "lecithin" was originally defined from the Greek lekithos (λεκιθος, egg yolk) by Theodore Nicolas Gobley, a French chemist and pharmacist of the mid-19th century, who applied it to the egg yolk phosphatidylcholine that he identified in 1847. Gobley eventually completely described his lecithin from chemical structural point of view, in 1874. Phosphatidylcholines are such a major component of lecithin that in some contexts the terms are sometimes used as synonyms. However, lecithin extracts consist of a mixture of phosphatidylcholine and other compounds. It is also used along with sodium taurocholate for simulating fed- and fasted-state biorelevant media in dissolution studies of highly lipophilic drugs.
Phosphatidylcholine is a major constituent of cell membranes and pulmonary surfactant, and is more commonly found in the exoplasmic or outer leaflet of a cell membrane. It is thought to be transported between membranes within the cell by phosphatidylcholine transfer protein (PCTP).[3]
Phosphatidylcholine also plays a role in membrane-mediated cell signaling and PCTP activation of other enzymes.[4]
Structure and physical properties

This phospholipid is composed of a choline head group and glycerophosphoric acid, with a variety of fatty acids. Usually, one is a saturated fatty acid (in the given figure, this can be palmitic or hexadecanoic acid, H3C-(CH2)14-COOH; margaric acid identified by Gobley in egg yolk, or heptadecanoic acid H3C-(CH2)15-COOH, also belong to that class); and the other is an unsaturated fatty acid (here oleic acid, or 9Z-octadecenoic acid, as in Gobley's original egg yolk lecithin). However, there are also examples of disaturated species. Animal lung phosphatidylcholine, for example, contains a high proportion of dipalmitoylphosphatidylcholine.[5]
Phospholipase D catalyzes the hydrolysis of phosphatidylcholine to form phosphatidic acid (PA), releasing the soluble choline headgroup into the cytosol.
Phosphatidylcholine is a neutral lipid, but it carries an electric dipole moment of about 10 D.[6] Vibrational dynamics of phosphatidylcholine and its hydration waters has been recently calculated from first principles.[7]
Possible health benefits
Senescence
Phosphatidylcholine is a vital substance found in every cell of the human body. Thus, some researchers have used mutant mouse models with severe oxidative damage as a model of "accelerated aging" to investigate the possible role of phosphatidylcholine supplementation as a way of slowing down aging-related processes[8] and improving brain functioning and memory capacity in dementia.[9] However, a systematic review of clinical trials in humans found that there is not enough evidence to support lecithin or phosphatidylcholine supplementation for patients with dementia. The study does admit that a moderate benefit cannot be ruled out until further large scale studies are performed.[10]
Liver repair
Recent studies have examined potential benefits of phosphatidylcholine for liver repair. Results are mixed in animal models,[11] and no clinical evidence shows a health benefit in humans. One study shows the healing effect of phosphatidylcholine in mice with hepatitis A, hepatitis B, and hepatitis C. The administration of phosphatidylcholine for chronic, active hepatitis resulted in significant reduction of disease activity in mice.[12]
Lipolysis
Some organizations promote the use of injected phosphatidylcholine, otherwise known as injection lipolysis, claiming the procedure can break down fat cells, and thus serve as an alternative to liposuction. While the procedure cites early experiments that showed lipolysis in cases of fat emboli,[13] no peer-reviewed studies have shown any amount of lipolysis even remotely comparable to liposuction.[14][15]
Ulcerative colitis
Phase IIa/b clinical trials performed at the Heidelberg University Hospital[16] have shown that delayed release purified phosphatidylcholine is an anti-inflammatory agent, and a surface hydrophobicity increasing compound with promising therapeutic potential in the treatment of inflammatory bowel disease.
Possible health risks
A report in 2011 has linked the microbial catabolites of phosphatidylcholine with increased atherosclerosis in mice through the production of choline, trimethylamine oxide, and betaine.[17]
See also
Additional images
-

General structural formula of phosphatidylcholines
-

Choline metabolism
References
- ↑ Jackowski, Suzanne; Cronan, jr., John E.; Rock, Charles O. (1991). "Chapter 2: Lipid metabolism in procaryotes". In Vance, Dennis E.; Vance, J. Biochemistry of Lipids, Lipoproteins and Membranes. Elsevier. pp. 80–81. ISBN 0-444-89321-0.
- ↑ Chen F, Zhao Q, Cai X, Lv L, Lin W, Yu X, Li C, Li Y, Xiong M, Wang XG (November 2009). "Phosphatidylcholine in membrane of Escherichia coli changes bacterial antigenicity.". Can. J. Microbiol. 55 (11): 1328–34. doi:10.1139/w09-082. PMID 19940943.
- ↑ Wirtz KW (July 1991). "Phospholipid transfer proteins.". Ann. Rev. Biochem. 60 (13): 73–99. doi:10.1146/annurev.bi.60.070191.000445. PMID 1883207.
- ↑ Kanno K, Wu MK, Agate DA, Fanelli BK, Wagle N, Scapa EF, Ukomadu C, Cohen DE (October 2007). "Interacting proteins dictate function of the minimal START domain phosphatidylcholine transfer protein/StarD2.". J. Biol. Chem. 282 (42): 30728–36. doi:10.1074/jbc.M703745200. PMID 17704541.
- ↑ http://lipidlibrary.aocs.org/Lipids/pc/index.htm
- ↑ Mashaghi et al. Journal of Chemical Physics 136, 114709 (2012)
- ↑ Tayebeh Jadidi et al 2013 1|Europhysics Letters 102 28008
- ↑ Mei-Chu Hung, Koji Shibasaki, Riki Yoshida, Masao Sato and Katsumi Imaizumi (2001). Learning behaviour and cerebral protein kinase C, antioxidant status, lipid composition in senescence-accelerated mouse: influence of a phosphatidylcholine–vitamin B12 diet. British Journal of Nutrition, 86, pp 163-171 doi:10.1079/BJN2001391
- ↑ Chung, Shu-Ying, Tomoe Moriyama, Eiko Uezu, Kayoko Uezu, Rieko Hirata, Noriko Yohena, Yasnunobu Masuda, Toyohiko Kokubu, and Shigeru Yamamoto. 1995. "Administration of Phosphatidylcholine Increases Brain Acetylcholine Concentration and Improves Memory in Mice with Dementia." The Journal of Nutrition 125: 1484-489. Print.
- ↑ Higgins, Julian; Leon Flicker (21 January 2009). Higgins, Julian PT, ed. "Lecithin for dementia and cognitive impairment". Cochrane Database of Systematic Reviews of Systematic Reviews 4 (3): CD001015. doi:10.1002/14651858.CD001015. PMID 12917896.
- ↑ Niebergall, LJ; Jacobs, RL; Chaba, T; Vance, DE (2011). "Phosphatidylcholine protects against steatosis in mice but not non-alcoholic steatohepatitis". Biochimica et Biophysica Acta 1811 (12): 1177–85. doi:10.1016/j.bbalip.2011.06.021. PMID 21745592.
- ↑ Sally Tandy; Rosanna WS Chung; Alvin Kamili; Elaine Wat; Jacquelyn M Weir; Peter J Meikle; Jeffrey S Cohn (30 November 2010). "Hydrogenated phosphatidylcholine supplementation reduces hepatic lipid levels in mice fed a high-fat diet". Atherosclerosis (Elsevier) 213 (1): 142–147. doi:10.1016/j.atherosclerosis.2010.07.050.
- ↑ Hasegawa, Toshio; Matsukura, Tomoyuki; Ikeda, Shigaku (2010). "Mesotherapy for Benign Symmetric Lipomatosis". Aesthetic Plastic Surgery 34 (2): 153–156. doi:10.1007/s00266-009-9374-4. PMID 19488808.
Intralesional injection, termed mesotherapy, using phosphatidylcholine is a potentially effective therapy for benign symmetric lipomatosis that should be reconsidered as a therapeutic option for this disease.
- ↑ Rotunda, Adam M.; Kolodney, Michael S. (2006). "Mesotherapy and Phosphatidylcholine Injections: Historical Clarification and Review". Dermatologic Surgery 32 (4): 465–480. doi:10.1111/j.1524-4725.2006.32100.x. PMID 16681654.
Recent laboratory investigations17 demonstrate that sodium deoxycholate, a bile salt also used as a laboratory detergent,102,103 was just as potent at causing adipocyte lysis and cell death as the complete phosphatidylcholine formula, which contains both phosphatidylcholine and deoxycholate (Figure 3). This bile salt is used to solubilize phosphatidylcholine by forming mixed micelles composed of phosphatidylcholine and deoxycholate.102,104 It is common practice to combine intravenous medications with bile salts to improve their water solubility.105,106 These findings suggest that sodium deoxycholate is the primary active ingredient in the phosphatidylchloline preparations.
- ↑ Park, Seung Ha; Kim, Deok Woo; Lee, Min Ah; Yoo, Sang Chul; Rhee, Seung Chul; Koo, Sang Hwan; Seol, Geun Hye; Cho, Eun Young (April 2008). "Effectiveness of Mesotherapy on Body Contouring". Plastic & Reconstructive Surgery 121 (4): 179e–185e. doi:10.1097/01.prs.0000304611.71480.0a. PMID 18349597.
The author, when discussing phosphatidylcholine as a part of mesotherapy concludes: 'Although there is a preliminary report contradictory to this result, there was no body contouring observed in this study. There were no statistically significant changes in thigh girth, cross-sectional area, or laboratory values for the lipid profile except for a decrease in the triglyceride level in the blood, which might be an indirect effect of the method of aminophylline absorption into the systemic circulation.'
- ↑ Schneider, H.; Braun, A.; Füllekrug, J.; Stremmel, W.; Ehehalt, R. (2010). "Lipid Based Therapy for Ulcerative Colitis—Modulation of Intestinal Mucus Membrane Phospholipids as a Tool to Influence Inflammation". International Journal of Molecular Sciences 11 (10): 4149–4164. doi:10.3390/ijms11104149. PMC 2996791. PMID 21152327.
- ↑ Wang Z; Klipfell, Elizabeth; Bennett, Brian J.; Koeth, Robert; Levison, Bruce S.; Dugar, Brandon; Feldstein, Ariel E.; Britt, Earl B.; et al. (April 2011). "Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease". Nature 472 (7341): 57–63. doi:10.1038/nature09922. PMC 3086762. PMID 21475195.
External links
- Phosphatidylcholines at the US National Library of Medicine Medical Subject Headings (MeSH)
- http://www.kvue.com/news/top/stories/110507kvuelipodissolve-mm.1e0189bdb.html
| ||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||