Phenyl group

In organic chemistry, the phenyl group or phenyl ring is a cyclic group of atoms with the formula C6H5. Phenyl groups are closely related to benzene and can be viewed as a benzene ring, minus a hydrogen, serving as a functional group. Phenyl groups have six carbon atoms bonded together in a hexagonal planar ring, five of which are bonded to individual hydrogen atoms, with the remaining carbon bonded to a substituent. Phenyl groups are commonplace in organic chemistry.[1] Although often depicted with alternating double and single bonds, phenyl groups are chemically aromatic and show nearly equal bond lengths between carbon atoms in the ring.[1][2]
Nomenclature
Usually, a "phenyl group" is synonymous to C6H5– and is represented by the symbol Ph or, archaically, Φ. Benzene is sometimes denoted as PhH. Phenyl groups are generally attached to other atoms or groups. For example, triphenylmethane (Ph3CH) has three phenyl groups attached to the same carbon center. Many or even most phenyl compounds are not described with the term "phenyl". For example the chloro derivative C6H5Cl is normally called chlorobenzene, although it could be called phenyl chloride. In special (and rare) cases, isolated phenyl groups are detected: the phenyl anion (C6H5−), the phenyl cation (C6H5+), and the phenyl radical (C6H5·).
Although Ph and phenyl uniquely denote C6H5, substituted derivatives also are described using the phenyl terminology. For example, O2NC6H4 is nitrophenyl (of which three isomers are possible) and F5C6 is pentafluorophenyl.
Etymology
Phenyl is derived from the French word phényle, which in turn derived from Greek phaino – "shining", as the first phenyl compounds named were byproducts of making and refining various gases used for lighting.[3]
Structure, bonding, characterization
Phenyl compounds are derived from benzene (C6H6), at least conceptually and often in terms of their production. In terms of its electronic properties, the phenyl group is related to a vinyl group. The phenyl group is hydrophobic. Phenyl groups tend to resist oxidation and reduction. Phenyl groups (like all aromatic compounds) have enhanced stability in comparison to equivalent bonding in aliphatic (non-aromatic) groups. This increased stability is due to the unique properties of aromatic molecular orbitals.[2]
The bond lengths between carbon atoms in a phenyl group are approximately 1.4 Å.[4]
In 1H-NMR spectroscopy, protons of a phenyl group typically have chemical shifts around 7.27 ppm. These chemical shifts are influenced by aromatic ring current and may change depending on substituents.
Preparation, occurrence, and applications
Phenyl groups are usually introduced using reagents that behave as sources of the phenyl anion or the phenyl cation. Representative reagents include phenyllithium (C6H5Li) and phenylmagnesium bromide (C6H5MgBr). Electrophiles attack benzene to give phenyl derivatives:
- C6H6 + E+ → C6H5E + H+
where E+ (the "electrophile") = Cl+, NO2+, SO3. These reactions are called electrophilic aromatic substitutions.
- Representative compounds containing phenyl groups
-
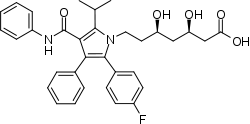
Atorvastatin (Lipitor), a blockbuster drug featuring two phenyl and one p-fluorophenyl groups. It is used to lower cholesterol in people with hypercholesterolaemia.
-

Fexofenadine (Allegra, Telfast), another blockbuster drug, which features a diphenylmethyl group as well as a p-phenylene (C6H4) group. It is an antihistamine used to treat allergies.
-

Phenylalanine, a common amino acid.
-
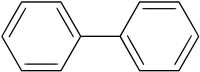
Biphenyl, consisting of two phenyl groups. The two rings tend not to be coplanar.
-

Chlorobenzene (or phenyl chloride), a solvent.
Phenyl groups are found in many organic compounds, both natural and synthetic (see figure). Most common among natural products is the amino acid phenylalanine, which contains a phenyl group. A major product of the petrochemical industry is "BTX" consisting of benzene, toluene, and xylene - all of which are building blocks for phenyl compounds. The polymer polystyrene is derived from a phenyl-containing monomer and owes its properties to the rigidity and hydrophobicity of the phenyl groups. Many drugs as well as many pollutants contain phenyl rings. biphe One of the simplest phenyl-containing compounds is phenol, C6H5OH. It is often said the resonance stability of phenol makes it a stronger acid than that of aliphatic alcohols such as ethanol (pKa = 10 vs. 16–18). However, a significant contribution is the greater electronegativity of the sp2 alpha carbon in phenol compared to the sp3 alpha carbon in aliphatic alcohols.[5]
References
- 1 2 March, J. “Advanced Organic Chemistry” 4th Ed. J. Wiley and Sons, 1992: New York. ISBN 0-471-60180-2.
- 1 2 "Virtual Textbook of Organic Chemistry: Aromaticity". Michigan State University. http://www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/react3.htm
- ↑ Oxford Dictionaries
- ↑ Hendrik F. Hameka. "Computation of the structures of the phenyl and benzyl radicals with the UHF method". J. Org. Chem., 1987, 52 (22), pp. 5025–5026. http://pubs.acs.org/doi/abs/10.1021/jo00231a035
- ↑ "Inductive and Resonance Effects on the Acidities of Phenol, Enols, and Carbonyl α-Hydrogens." Pedro J. Silva J. Org. Chem. 2009 (Solvation effects on the relative acidities of acetaldehyde enol and phenol described in the Supporting Information)
|