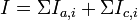Partial current
In electrochemistry, partial current is defined as the electric current associated with (anodic or cathodic) half of the electrode reaction.
Depending on the electrode half-reaction, one can distinguish two types of partial current:
- cathodic partial current Ic (called also cathodic current): is the flow of electrons from the electrode surface to a species in solution;
- anodic partial current Ia (called also anodic current): is the flow of electrons into the electrode from a species in solution.
The cathodic and anodic partial currents are defined by IUPAC.[1]
The partial current densities (ic and ia) are the ratios of partial currents respect to the electrode areas (Ac and Aa):
- ic = Ic/Ac
- ia = Ia/Aa
The sum of the cathodic partial current density ic (positive) and the anodic partial current density ia (negative) gives the net current density i:[2]
- i = ic + ia
In the case of the cathodic partial current density being equal to the anodic partial current density (for example, in a corrosion process[3]), the net current density on the electrode is zero:[2]
- ieq = ic,eq + ia,eq = 0
When more than one reaction occur on an electrode simultaneously, then the total electrode current can be expressed as:[1]
where the index i refers to the particular reactions.
Notes
- 1 2 http://goldbook.iupac.org/P04407.html
- 1 2 Electrochemistry Dictionary and Encyclopedia
- ↑ B. Elsener, Corrosion and durability of metals , p. 252.
References
- Bard, A.J. and Faulkner L.R. Electrochemical Methods: Fundamentals and Applications (2nd ed.), 2001 John Wiley & Sons Inc.