Reduction potential
Reduction potential (also known as redox potential, oxidation / reduction potential, ORP, pE, ε, or 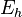 ) is a measure of the tendency of a chemical species to acquire electrons and thereby be reduced. Reduction potential is measured in volts (V), or millivolts (mV). Each species has its own intrinsic reduction potential; the more positive the potential, the greater the species' affinity for electrons and tendency to be reduced. ORP is a common measurement for water quality.[1]
) is a measure of the tendency of a chemical species to acquire electrons and thereby be reduced. Reduction potential is measured in volts (V), or millivolts (mV). Each species has its own intrinsic reduction potential; the more positive the potential, the greater the species' affinity for electrons and tendency to be reduced. ORP is a common measurement for water quality.[1]
Measurement and interpretation
In aqueous solutions, reduction potential is a measure of the tendency of the solution to either gain or lose electrons when it is subject to change by introduction of a new species. A solution with a higher (more positive) reduction potential than the new species will have a tendency to gain electrons from the new species (i.e. to be reduced by oxidizing the new species) and a solution with a lower (more negative) reduction potential will have a tendency to lose electrons to the new species (i.e. to be oxidized by reducing the new species). Because the absolute potentials are difficult to accurately measure, reduction potentials are defined relative to a reference electrode. Reduction potentials of aqueous solutions are determined by measuring the potential difference between an inert sensing electrode in contact with the solution and a stable reference electrode connected to the solution by a salt bridge.[2]
The sensing electrode acts as a platform for electron transfer to or from the reference half cell. It is typically platinum, although gold and graphite can be used. The reference half cell consists of a redox standard of known potential. The standard hydrogen electrode (SHE) is the reference from which all standard redox potentials are determined and has been assigned an arbitrary half cell potential of 0.0 mV. However, it is fragile and impractical for routine laboratory use. Therefore, other more stable reference electrodes such as silver chloride and saturated calomel (SCE) are commonly used because of their more reliable performance.
Although measurement of the reduction potential in aqueous solutions is relatively straightforward, many factors limit its interpretation, such as effects of solution temperature and pH, irreversible reactions, slow electrode kinetics, non-equilibrium, presence of multiple redox couples, electrode poisoning, small exchange currents and inert redox couples. Consequently, practical measurements seldom correlate with calculated values. Nevertheless, reduction potential measurement has proven useful as an analytical tool in monitoring changes in a system rather than determining their absolute value (e.g. process control and titrations).
Explanation
Just as the transfer of hydrogen ions between chemical species determines the pH of an aqueous solution, the transfer of electrons between chemical species determines the reduction potential of an aqueous solution. Like pH, the reduction potential represents how strongly electrons are transferred to or from species in solution. It does not characterize the amount of electrons available for oxidation or reduction, in much the same way that pH does not characterize the buffering capacity.
In fact, it is possible to define pE, the negative logarithm of electron concentration (-log[e]) in a solution, which will be directly proportional to the redox potential.[2][3] Sometimes pE is used as a unit of reduction potential instead of Eh, for example in environmental chemistry.[2] If we normalize pE of hydrogen to zero, we will have the relation pE=16.9 Eh at room temperature. This point of view is useful for understanding redox potential, although the transfer of electrons, rather than the absolute concentration of free electrons in thermal equilibrium, is how one usually thinks of redox potential. Theoretically, however, the two approaches are equivalent.
Conversely, one could define a potential corresponding to pH as a potential difference between a solute and pH neutral water, separated by porous membrane (that is permeable to hydrogen ions). Such potential differences actually do occur from differences in acidity on biological membranes. This potential (where pH neutral water is set to 0V) is analogous with redox potential (where standardized hydrogen solution is set to 0V), but instead of hydrogen ions, electrons are transferred across in the redox case. Both pH and redox potentials are properties of solutions, not of elements or chemical compounds per se, and depend on concentrations, temperature etc.
Standard reduction potential
The standard reduction potential ( ) is measured under standard conditions: 25°C, a 1 activity for each ion participating in the reaction, a partial pressure of 1 bar for each gas that is part of the reaction, and metals in their pure state. The standard reduction potential is defined relative to a standard hydrogen electrode (SHE) reference electrode, which is arbitrarily given a potential of 0.00 volts. Historically, many countries, including the United States and Canada, used standard oxidation potentials rather than reduction potentials in their calculations. These are simply the negative of standard reduction potentials, so it is not a major problem in practice. However, because these can also be referred to as "redox potentials", the terms "reduction potentials" and "oxidation potentials" are preferred by the IUPAC. The two may be explicitly distinguished in symbols as
) is measured under standard conditions: 25°C, a 1 activity for each ion participating in the reaction, a partial pressure of 1 bar for each gas that is part of the reaction, and metals in their pure state. The standard reduction potential is defined relative to a standard hydrogen electrode (SHE) reference electrode, which is arbitrarily given a potential of 0.00 volts. Historically, many countries, including the United States and Canada, used standard oxidation potentials rather than reduction potentials in their calculations. These are simply the negative of standard reduction potentials, so it is not a major problem in practice. However, because these can also be referred to as "redox potentials", the terms "reduction potentials" and "oxidation potentials" are preferred by the IUPAC. The two may be explicitly distinguished in symbols as 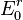 and
and 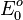 .
.
Converting potentials between different types of reference electrodes
Often a reduction potential is quoted as measured against a different reference electrode than the one desired and it becomes necessary to convert to the desired reference potential. Alternatively, it may be necessary to convert measurements to the standard reduction potential for reporting purposes. This is easily done by recognizing that the observed potential represents the difference between the potential at the sensing electrode and the potential at the reference electrode, i.e.
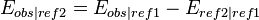
Where 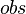 is the observed reaction,
is the observed reaction, 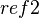 is the reference used in experiment, and
is the reference used in experiment, and 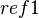 is the reference that is desired. The voltage relationships for several different reference electrodes at 25 °C can be interrelated as follows:
is the reference that is desired. The voltage relationships for several different reference electrodes at 25 °C can be interrelated as follows:
| Reference electrode | Electrode potential with respect to SHE (mV) |
|---|---|
| Standard hydrogen electrode (SHE) | 0 |
| Saturated calomel electrode (SCE) | +241 |
| Ag/AgCl, 1 M KCl | +192 |
| Ag/AgCl, 4 M KCl | +228 |
| Ag/AgCl, sat. KCl | +236 |
For example, if one measured 300 mV using a saturated KCl Ag/AgCl reference(ref2) and wanted to refer it to the standard reduction potential ( ) measured using a SHE reference electrode (ref1), then 192 mV should be added to the 300 mV to obtain 492 mV, since
) measured using a SHE reference electrode (ref1), then 192 mV should be added to the 300 mV to obtain 492 mV, since
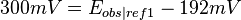
it follows that
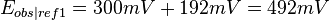
and therefore
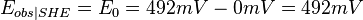
Likewise, if one measured 300 mV using a saturated KCl Ag/AgCl reference (ref2) and wanted to determine the corresponding measurement using an SCE reference (ref1), then given
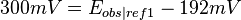
it follows that
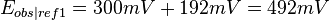
and therefore
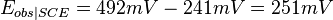
Half cells
The relative reactivities of different half cells can be compared to predict the direction of electron flow. A higher  means there is a greater tendency for reduction to occur, while a lower one means there is a greater tendency for oxidation to occur.
means there is a greater tendency for reduction to occur, while a lower one means there is a greater tendency for oxidation to occur.
Any system or environment that accepts electrons from a normal hydrogen electrode is a half cell that is defined as having a positive redox potential; any system donating electrons to the hydrogen electrode is defined as having a negative redox potential. 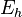 is measured in millivolts (mV). A high positive
is measured in millivolts (mV). A high positive 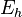 indicates an environment that favors oxidation reaction such as free oxygen. A low negative
indicates an environment that favors oxidation reaction such as free oxygen. A low negative 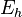 indicates a strong reducing environment, such as free metals.
indicates a strong reducing environment, such as free metals.
Sometimes when electrolysis is carried out in an aqueous solution, water, rather than the solute, is oxidized or reduced. For example, if an aqueous solution of NaCl is electrolyzed, water may be reduced at the cathode to produce H2(g) and OH− ions, instead of Na+ being reduced to Na(s), as occurs in the absence of water. It is the reduction potential of each species present that will determine which species will be oxidized or reduced.
Absolute reduction potentials can be determined if we find the actual potential between electrode and electrolyte for any one reaction. Surface polarization interferes with measurements, but various sources give an estimated potential for the standard hydrogen electrode of 4.4 V to 4.6 V (the electrolyte being positive.)
Half-cell equations can be combined if one is reversed to an oxidation in a manner that cancels out the electrons to obtain an equation without electrons in it.
Nernst equation
The 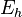 and pH of a solution are related. For a half cell equation, conventionally written as reduction (electrons on the left side):
and pH of a solution are related. For a half cell equation, conventionally written as reduction (electrons on the left side):
![a A + b B + n [e^{-}] + h [H^{+}] = c C + d D](../I/m/f61f1e98ccb63fd12df0f2691eef7194.png)
The half cell standard potential  is given by:
is given by:
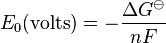
where 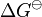 is the standard Gibbs free energy change,
is the standard Gibbs free energy change,  is the number of electrons involved, and
is the number of electrons involved, and 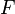 is Faraday's constant. The Nernst equation relates pH and
is Faraday's constant. The Nernst equation relates pH and 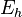 :
:
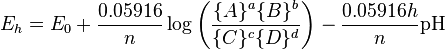
where curly brackets indicate activities and exponents are shown in the conventional manner. This equation is the equation of a straight line for 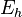 as a function of pH with a slope of
as a function of pH with a slope of 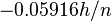 volt (pH has no units.) This equation predicts lower
volt (pH has no units.) This equation predicts lower 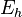 at higher pH values. This is observed for reduction of O2 to OH− and for reduction of H+ to H2. If H+ were on the opposite side of the equation from H+, the slope of the line would be reversed (higher
at higher pH values. This is observed for reduction of O2 to OH− and for reduction of H+ to H2. If H+ were on the opposite side of the equation from H+, the slope of the line would be reversed (higher 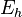 at higher pH).
An example of that would be the formation of magnetite (Fe3O4) from HFeO−
at higher pH).
An example of that would be the formation of magnetite (Fe3O4) from HFeO−
2 (aq):[4]
3 HFeO−
2 + H+ = Fe3O4 + 2 H2O + 2 [[e<sup>−</sup>]]
where 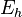 = −1.1819 − 0.0885 log([HFeO−
= −1.1819 − 0.0885 log([HFeO−
2]3) + 0.0296 pH. Note that the slope of the line is −1/2 the −0.05916 value above, since 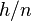 = −1/2.
= −1/2.
Biochemistry
Many enzymatic reactions are oxidation-reduction reactions in which one compound is oxidized and another compound is reduced. The ability of an organism to carry out oxidation-reduction reactions depends on the oxidation-reduction state of the environment, or its reduction potential (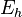 ).
).
Strictly aerobic microorganisms are generally active at positive 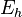 values, whereas strict anaerobes are generally active at negative
values, whereas strict anaerobes are generally active at negative 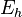 values. Redox affects the solubility of nutrients, especially metal ions.[5]
values. Redox affects the solubility of nutrients, especially metal ions.[5]
There are organisms that can adjust their metabolism to their environment, such as facultative anaerobes. Facultative anaerobes can be active at positive Eh values, and at negative Eh values in the presence of oxygen bearing inorganic compounds, such as nitrates and sulfates.
Environmental chemistry
In the field of environmental chemistry, the reduction potential is used to determine if oxidizing or reducing conditions are prevalent in water or soil, and to predict the states of different chemical species in the water, such as dissolved metals. pE values in water range from -12 to 25; the levels where the water itself becomes reduced or oxidized, respectively.[2]
The reduction potentials in natural systems often lie comparatively near one of the boundaries of the stability region of water. Aerated surface water, rivers, lakes, oceans, rainwater and acid mine water, usually have oxidizing conditions (positive potentials). In places with limitations in air supply, such as submerged soils, swamps and marine sediments, reducing conditions (negative potentials) are the norm. Intermediate values are rare and usually a temporary condition found in systems moving to higher or lower pE values.[2]
In environmental situations, it is common to have complex non-equilibrium conditions between a large number of species, meaning that it is often not possible to make accurate and precise measurements of the reduction potential. However, it is usually possible to obtain an approximate value and define the conditions as being in the oxidizing or reducing regime.[2]
Water quality
Oxidation reduction potential (ORP) can be used for water system monitoring with the benefit of a single-value measure of the disinfection potential, showing the activity of the disinfectant rather than the applied dose.[1] For example, E. coli, Salmonella, Listeria and other pathogens have survival times of under 30 s when the ORP is above 665 mV, compared against >300 s when it is below 485 mV.[1]
A study was conducted comparing traditional parts per million chlorination reading and ORP in Hennepin County, Minnesota. The results of this study argue for the inclusion of ORP above 650mV in local health codes.[6]
Geology
Eh-pH (Pourbaix) diagrams are commonly used in mining and geology for assessment of the stability fields of minerals and dissolved species. Under conditions where a mineral (solid) phase is the most stable form of an element, these diagrams show that mineral. As with results from all thermodynamic (equilibrium) evaluations, these diagrams should be used with caution. Although the formation of a mineral or its dissolution may be predicted to occur under a set of conditions, the process may be negligible because its rate is so slow. Under those circumstances, kinetic evaluations are necessary. However, the equilibrium conditions can be used to evaluate the direction of spontaneous changes and the magnitude of the driving force behind them.
See also
- Galvanic cell
- Electrolytic cell
- Electromotive force
- Electrochemical potential
- Standard electrode potential
- Oxygen radical absorbance capacity
- Redox
References
- 1 2 3 Trevor V. Suslow, 2004, Oxidation-Reduction Potential for Water Disinfection Monitoring, Control, and Documentation, University of California Davis, http://anrcatalog.ucdavis.edu/pdf/8149.pdf
- 1 2 3 4 5 6 vanLoon, Gary; Duffy, Stephen (2011). Environmental Chemistry - a global perspective (3rd ed.). Oxford University Press. pp. 235–248. ISBN 978-0-19-922886-7.
- ↑ 1981 Stumm, W. and Morgan, J. J. (1981): Aquatic chemistry, 2nd Ed.; John Wiley & Sons, New York
- ↑ Garrels, R.M.; Christ, C.L. (1990). Minerals, Solutions, and Equilibria. London: Jones and Bartlett.
- ↑ Chuan, M.; Liu, G. Shu. J (1996). "Solubility of heavy metals in a contaminated soil: Effects of redox potential and pH". Water, Air, & Soil Pollution. doi:10.1007/BF00282668.
- ↑ Bastian, Tiana; Brondum, Jack. "Do Traditional Measures of Water Quality in Swimming Pools and Spas Correspond with Beneficial Oxidation Reduction Potential?". Retrieved 21 January 2014.
Additional notes
Onishi, j; Kondo W; Uchiyama Y (1960). "Preliminary report on the oxidation-reduction potential obtained on surfaces of gingiva and tongue and in interdental space.". Bull Tokyo Med Dent Univ (7): 161.
External links
- Redox potential definition
- Large table of potentials (Site broken. Archived version on the Internet Archive.)