Oxibendazole
 | |
| Systematic (IUPAC) name | |
|---|---|
|
methyl N-(6-propoxy-1H-benzimidazol-2-yl)carbamate | |
| Clinical data | |
| AHFS/Drugs.com | International Drug Names |
| Legal status |
|
| Identifiers | |
| CAS Number |
20559-55-1 |
| ATCvet code | QP52AC07 |
| PubChem | CID 4622 |
| ChemSpider |
4461 |
| UNII |
022N12KJ0X |
| KEGG |
D05293 |
| Chemical data | |
| Formula | C12H15N3O3 |
| Molar mass | 249.26 g/mol |
| |
| |
| | |
Oxibendazole is a benzimidazole drug that is used to protect against roundworms, strongyles, threadworms, pinworms and lungworm infestations in horses and some domestic pets. It is usually white to yellowish in appearance, and may take the form of a powder or a tablet.
Synthesis
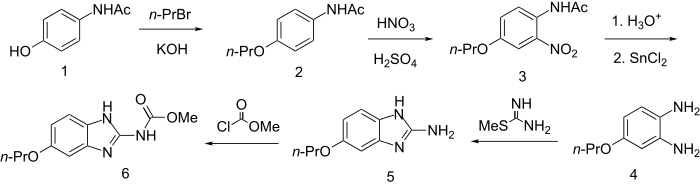
4-Hydroxyacetamide is alkylated with n-propylbromide in the presence of KOH to give the ether (2). Nitration of the product with HNO3/H2SO4 proceeds at the position ortho to the amido group (3); saponification followed by reduction of the nitro group with SnCl2gives the phenylenediamine (4).
Reaction of that intermediate with S-methylthiourea can be visualized as proceeding first to the guanidine obtained by addition to the imine double bond followed by elimination of methyl mercaptan. Cyclization completes the construction of the 2-aminobenzimidazole system. Acylation with methyl chloroformate results in the formation of a urethane on the amino group to produce 6.
References
- ↑ GB 1123317 corresp to P. P. Actor, J. F. Pagano, U.S. Patent 3,574,845 (1968, 1971 both to SKF).