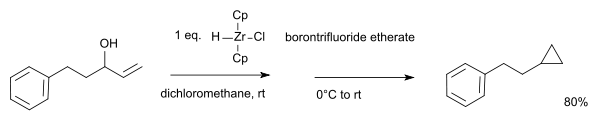Organozirconium chemistry

Organozirconium compounds are organometallic compounds containing a carbon to zirconium chemical bond. Organozirconium chemistry is the corresponding science exploring properties, structure and reactivity of these compounds.[1] In general organozirconium compounds are stable and non-toxic. They are used in organic chemistry as an intermediate in the synthesis of chemical compounds and share characteristics with organotitanium compounds also a Group 4 element. Organozirconium compounds have been widely studied, in part because they are useful catalysts in Ziegler-Natta polymerization.
The first organozirconium compound discovered (1953) was zirconocene dibromide,[2] belongs to the metallocene family. It was prepared in a reaction of the cyclopentadienyl magnesium bromide and zirconium(IV) chloride. Zirconocenes are used as polymerization catalysts such as Kaminsky catalysts, partly replacing organotitanium compounds.
Hydrozirconation
The so-called Schwartz's reagent (1974) is a zirconocene hydrochloride and a reagent in hydrometalation reactions (called hydrozirconation) with some use in organic synthesis. Substrates for hydrozirconation are alkenes and alkynes. With terminal alkynes the terminal vinyl zirconium product is predominantly formed. Secondary reactions are nucleophilic additions, transmetalations,[3] conjugate additions,[4] coupling reactions, carbonylation and halogenation.
History
The development of zirconium hydrides obviously preceded that of hydrozirconation. The first such hydride, Cp2ZrH2, was developed in 1966 by M.G.H. Wallbridge in a reaction of (Cp)2Zr(BH4)2 with triethylamine in benzene as an uneventful insoluble solid.[5] In 1970 H. Weigold and P.C. Wailes prepared the hydrochloride from the dichloride (Cp2ZrCl2) and Lithium aluminium hydride (or the related LiAlH(t-BuO)3).[6] They went on to investigate the reaction of these novel hydrides with carboxylic acids[7] (for example to compounds like CpZr(OCOR)3) and in 1971 then arrived at their reactions with alkynes.[8]
For example, with one equivalent of Cp2ZrClH they obtained from diphenylacetylene the corresponding alkenylzirconium as a mixture of cis and trans isomer. With two equivalents of hydride the endproduct was a mixture of erythro and threo zircono alkanes:
In 1974 Donald W. Hart and Jeffrey Schwartz realized how these compounds could be used in organic synthesis by reacting the organozirconium intermediates with electrophiles such as hydrochloric acid, bromine and acid chlorides to the corresponding alkane, bromoalkanes and ketones:[9]
The corresponding organoboron and organoaluminum compounds were already known but these are air-sensitive and/or pyrophoric whereas organozirconium compounds are not.
Scope
In one study the usual regioselectivity of an alkyne hydrozirconation is reversed with the addition of zinc chloride:[10][11]
One example of a one-pot hydrozirconation - carbonylation - coupling is depicted below:[12][13]
With certain allyl alcohols, the alcohol group is replaced by nucleophilic carbon forming a cyclopropane ring:[14]
Zirconocyclisation
Zirconocene dichloride can be used to cyclise enynes and dienes to give cyclic or bicyclic aliphatic systems.[15][16][17][18]
Organohafnium chemistry
Organohafnium compounds are nearly identical to organozirconium compounds in that these two metals are extremely similar chemically. Cationic hafnocene complexes are relevant to alpha-olefin polymerization. Typical catalysts are bis(cyclopentadienyl)hafnium(IV) dichloride, bis(cyclopentadienyl)hafnium(IV) dihydride, and dimethylbis(cyclopentadienyl)hafnium(IV).
See also
- Other chemistries of carbon with other elements in the periodic table.
| CH | He | ||||||||||||||||
| CLi | CBe | CB | CC | CN | CO | CF | Ne | ||||||||||
| CNa | CMg | CAl | CSi | CP | CS | CCl | CAr | ||||||||||
| CK | CCa | CSc | CTi | CV | CCr | CMn | CFe | CCo | CNi | CCu | CZn | CGa | CGe | CAs | CSe | CBr | CKr |
| CRb | CSr | CY | CZr | CNb | CMo | CTc | CRu | CRh | CPd | CAg | CCd | CIn | CSn | CSb | CTe | CI | CXe |
| CCs | CBa | CHf | CTa | CW | CRe | COs | CIr | CPt | CAu | CHg | CTl | CPb | CBi | CPo | CAt | Rn | |
| Fr | CRa | Rf | Db | CSg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | |
| ↓ | |||||||||||||||||
| CLa | CCe | CPr | CNd | CPm | CSm | CEu | CGd | CTb | CDy | CHo | CEr | CTm | CYb | CLu | |||
| Ac | CTh | CPa | CU | CNp | CPu | CAm | CCm | CBk | CCf | CEs | Fm | Md | No | Lr | |||
| Core organic chemistry | Many uses in chemistry |
| Academic research, but no widespread use | Bond unknown |
References
- ↑ A. Maureen Rouhi (19 April 2004). "Organozirconium Chemistry Arrives". Chemical & Engineering News 82 (16): 36–39. doi:10.1021/cen-v082n015.p035. ISSN 0009-2347.
- ↑ G. Wilkinson; P. L. Pauson; J. M. Birmingham; F. A. Cotton (1953). "Bis-cyclopentadienyl derivatives of some transition elements". Journal of the American Chemical Society 75 (4): 1011–1012. doi:10.1021/ja01100a527.
- ↑ "Allylic alcohols by alkene transfer from zirconium to zinc: 1-[(tert-butyldiphenylsilyl)oxy]-dec-3-en-5-ol". Organic Syntheses 9 (74): 205. 1998. Retrieved 2013-03-23.
Organic Syntheses, Coll. Vol. 9, p.143 (1998); Vol. 74, p.205 (1997).
- ↑ Conjugate Addition Of A Vinylzirconium Reagent: 3-(1-Octen-1-Yl)Cyclopentanone, Organic Syntheses, Coll. Vol. 9, p.640 (1998); Vol. 71, p.83 (1993).
- ↑ James, B. D.; Nanda, R. K.; Walbridge, M. G. H. (1967). "Reactions of Lewis bases with tetrahydroborate derivatives of the Group IVa elements. Preparation of new zirconium hydride species". Inorganic Chemistry 6 (11): 1979. doi:10.1021/ic50057a009.
- ↑ Wailes, P. C.; Weigold, H. (1970). "Hydrido complexes of zirconium I. Preparation". Journal of Organometallic Chemistry 24 (2): 405. doi:10.1016/S0022-328X(00)80281-8.
- ↑ Wailes, P. C.; Weigold, H. (1970). "Hydrido complexes of zirconium II. Reactions of dicyclopentadienylzirconium dihydride with carboxylic acids". Journal of Organometallic Chemistry 24 (2): 413. doi:10.1016/S0022-328X(00)80282-X.
- ↑ Wailes, P. C.; Weigold, H.; Bell, A. P. (1971). "Hydrido complexes of zirconium". Journal of Organometallic Chemistry 27 (3): 373. doi:10.1016/S0022-328X(00)82168-3.
- ↑ Bertin, J.; Kagan, H. B.; Luche, J. L.; Setton, R. (1974). "Graphite electrolytic lamellar reagents in organic chemistry. Esterifications in the presence of graphite bisulfate". Journal of the American Chemical Society 96 (26): 8113. doi:10.1021/ja00833a047.
- ↑ Directed Hydrozirconation of Propargylic Alcohols Donghui Zhang and Joseph M. Ready J. Am. Chem. Soc., 129 (40), 12088 -12089, 2007. doi:10.1021/ja075215o
- ↑ The electrophile in this reaction is iodine. The additive is believed to promote kinetic reaction control.
- ↑ Palladium-catalyzed coupling reaction of acylzirconocene chlorides with hypervalent iodonium salts: synthesis of aryl-substituted ketones Suk-Ku Kang and Seok-Keun Yoon J. Chem. Soc., Perkin Trans. 1, 2002, 459 - 461, doi:10.1039/b110983a
- ↑ Reagents: phenylacetylene, Schwartz's reagent, tetraphenylpalladium and the periodinane diphenyliodoniumtetrafluoroborate (phenyl group donor)
- ↑ A one-pot access to cyclopropanes from allylic ethers via hydrozirconation–deoxygenative ring formation Vincent Gandon, Jan Szymoniak, Chem. Commun., 2002, (12),1308-1309 doi: 10.1039/b203762a
- ↑ Fillery, Shaun F.; Richard J. Whitby; George J. Gordon; Tim Luker (1997). "Tandem reactions on a zirconocene template" (PDF). Pure & Applied Chemistry 69 (3): 633–638. doi:10.1351/pac199769030633. Retrieved 2013-03-23.
- ↑ Kasatkin, A.; Whitby, R. J. (1999). "Insertion of 1-Chloro-1-lithioalkenes into Organozirconocenes. A Versatile Synthesis of Stereodefined Unsaturated Systems". Journal of the American Chemical Society 121 (30): 7039. doi:10.1021/ja9910208.
- ↑ "Zirconium-promoted Bicyclization of Enynes". Comprehensive Organic Synthesis. 1991. pp. 1163–1184. doi:10.1016/B978-0-08-052349-1.00149-9. ISBN 978-0-08-052349-1.
- ↑ Whitby, R. J.; Dixon, S.; Maloney, P. R.; Delerive, P.; Goodwin, B. J.; Parks, D. J.; Willson, T. M. (2006). "Identification of Small Molecule Agonists of the Orphan Nuclear Receptors Liver Receptor Homolog-1 and Steroidogenic Factor-1". Journal of Medicinal Chemistry 49 (23): 6652–6655. doi:10.1021/jm060990k. PMID 17154495.
- ↑ Thomas, E.; Dixon, S.; Whitby, R. J. (2006). "A Rearrangement to a Zirconium–Alkenylidene in the Insertion of Dihalocarbenoids and Acetylides into Zirconacycles". Angewandte Chemie International Edition 45 (42): 7070. doi:10.1002/anie.200602822.