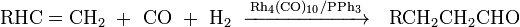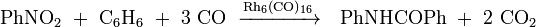Organorhodium chemistry
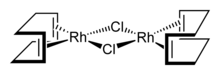
Organorhodium chemistry is the chemistry of organometallic compounds containing a rhodium-carbon chemical bond, and the study of rhodium and rhodium compounds as catalysts in organic reactions.[1]
Stable organorhodium compounds and transient organorhodium intermediates are used as catalyst such as in olefin hydroformylation, olefin hydrogenation, olefin isomerization and the Monsanto process[2]
Description
Organometallic rhodium compounds share many characteristics with those of cobalt (see organocobalt compounds), which is also in group 9. Rhodium can exist in oxidation states of -III to +IV, but rhodium(I) and rhodium(III) are the most common. Rhodium(I) compounds (d8 configuration) can occur with square planar or trigonal bipyramidal geometries, while rhodium (III) compounds (d6 configuration) typically have an octahedral geometry.[2]
Examples
Important homoleptic rhodium compounds are tetrarhodium dodecacarbonyl Rh4(CO)10 and hexadecacarbonylhexarhodium Rh6(CO)16. The hexarhodium compound is less preferred due to poor solubility. Both are important catalysts in hydroformylation of alkenes often accompanied by a phosphine ligand:
Nitrobenzene reduction is another reaction catalysed by this compound type:
Cyclooctadiene rhodium chloride dimer [RhCl(COD)]2 is investigated for its use in C-H bond activation. Sandwich compounds of rhodium, such as rhodocene, and half-sandwich compounds like [(η5-Cp)Rh(CO)2] are well known.
Another hexarhodium complex is (C60)Rh6(C60), where the rhodium center has two large fullerene ligands around it.
| Name | Molecular formula | CAS |
|---|---|---|
| (Acetylacetonato)dicarbonylrhodium(I) | Rh(CO)2(C5H7O2) | 14874-82-9 |
| Bicyclo[2.2.1]hepta-2,5-diene-rhodium(I) chloride dimer | C14H16Cl2Rh2 | 12257-42-0 |
| Chloro(1,5-cyclooctadiene)rhodium(I) dimer | C16H24Cl2Rh2 | 12092-47-6 |
| (Acetylacetonato)(norbornadiene)rhodium(I) | C12H15O22Rh | 32354-50-0 |
| Chloro(1,5-hexadiene)rhodium(I),dimer | C12H24Rh2Cl2 | 32965-49-4 |
| Acetylacetonatobis(ethylene)rhodium(I) | C9H15O2Rh | 12082-47-2 |
| [1,4-Bis(diphenylphosphino)butane](1,5-cyclooctadiene)rhodium(I) tetrafluoroborate | C36H40BF4P2Rh | 79255-71-3 |
Cyclometallation
Cyclometalated rhodium compounds constitute an important class of organometallic chemistry. Although such compounds are well documented in the literature rhodium(III) cyclometalates with azo function are spare. A typical example of this category viz. novel hexacoordinated orthometalated rhodium(III) thiolato complex trans-[Rh(C∧N∧S)Cl(PPh3)2] was synthesized from benzyl 2-(phenylazo)phenyl thioether and RhCl3·3H2O in the presence of excess PPh3 via in situ C(sp2)−H and C(sp3)−S bond scissions. This is the first example for a coordination compound of (phenylazo)thiolate ligand. The mechanism of formation of orthometalated azobenzene derivative was described to proceed via initial coordination of azo-nitrogen followed by electrophilic substitution at the pendant phenyl ring. PPh3 plays a crucial role in the C(sp3)−S cleavage process. Reductive cleavage by single electron transfer (SET) mechanism is likely to be operative for the C−S bond cleavage. Unlike analogous (phenylazo)phenolato compound the orthometalated thiolato complex exhibits a fully reversible oxidative wave at 0.82 V vs Ag/AgCl and this response is supposed to be primarily centered on the thiolato sulfur atom.[3]
See also
- Chemical bonds of carbon with other elements in the periodic table:
| CH | He | ||||||||||||||||
| CLi | CBe | CB | CC | CN | CO | CF | Ne | ||||||||||
| CNa | CMg | CAl | CSi | CP | CS | CCl | CAr | ||||||||||
| CK | CCa | CSc | CTi | CV | CCr | CMn | CFe | CCo | CNi | CCu | CZn | CGa | CGe | CAs | CSe | CBr | CKr |
| CRb | CSr | CY | CZr | CNb | CMo | CTc | CRu | CRh | CPd | CAg | CCd | CIn | CSn | CSb | CTe | CI | CXe |
| CCs | CBa | CHf | CTa | CW | CRe | COs | CIr | CPt | CAu | CHg | CTl | CPb | CBi | CPo | CAt | Rn | |
| Fr | CRa | Rf | Db | CSg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | |
| ↓ | |||||||||||||||||
| CLa | CCe | CPr | CNd | CPm | CSm | CEu | CGd | CTb | CDy | CHo | CEr | CTm | CYb | CLu | |||
| Ac | CTh | CPa | CU | CNp | CPu | CAm | CCm | CBk | CCf | CEs | Fm | Md | No | Lr | |||
| Core organic chemistry | Many uses in chemistry |
| Academic research, but no widespread use | Bond unknown |
References
- ↑ Synthesis of Organometallic Compounds: A Practical Guide Sanshiro Komiya Ed. S. Komiya, M. Hurano 1997
- 1 2 Crabtree, Robert H. (2005). The Organometallic Chemistry of the Transition Metals (4th ed.). USA: Wiley-Interscience. ISBN 0-471-66256-9.
- ↑ K. Pramanik, U. Das, B. Adhikari, D. Chopra, H. Stoeckli-Evans (2008). "RhCl3-Assisted C-H and C-S Bond Scissions: Isomeric Self-Association of Organorhodium(III) Thiolato Complex. Synthesis, Structure, and Electrochemistry". Inorg. Chem. 47 (2): 429–438. doi:10.1021/ic7016006. PMID 18161963.