Organoaluminium chemistry

Organoaluminium chemistry is the study of compounds containing bonds between carbon and aluminium bond. It is one of the major themes within organometallic chemistry.[1][2] Illustrative organoaluminium compounds are the dimer trimethylaluminium, the monomer triisobutylaluminium, and the titanium-aluminium compound called Tebbe's reagent. The behavior of organoaluminium compounds can be understood in terms of the polarity of the C−Al bond and the high Lewis acidity of the three-coordinated species. Industrially, these compounds are mainly used for the production of polyolefins.
History
The first organoaluminium compound (C2H5)3Al2I3 was discovered in 1859.[3] Organoaluminium compounds were however little known until the 1950s when Karl Ziegler and colleagues discovered the direct synthesis of trialkylaluminium compounds and applied these compounds to catalytic olefin polymerization. This line of research ultimately resulted in the Nobel Prize to Ziegler.
Structure and bonding
Aluminium(III) compounds
Organoaluminium compounds generally feature three- and four-coordinate Al centers, although higher coordination numbers are observed with inorganic ligands such as fluoride. In accord with the usual trends, four-coordinate Al prefers to be tetrahedral. In contrast to boron, aluminium is a larger atom and easily accommodates four carbon ligands. The triorganoaluminium compounds are thus usually dimeric with a pair of bridging alkyl ligands, e.g., Al2(C2H5)4(μ-C2H5)2. Thus, despite its common name of triethylaluminium, this compound contains two aluminium centres, and six ethyl groups. When the organoaluminium compound contain hydride or halide, these smaller ligands tend to occupy the bridging sites. Three coordination occurs when the R groups is bulky, e.g. Al(Mes)3 (Mes = 2,4,6-Me3C6H2 or mesityl) or isobutyl.[4]
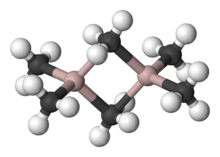
Ligand exchange in trialkylaluminium compounds
The trialkylaluminium dimers often participate in dynamic equilibria, resulting in the interchange of bridging and terminal ligands as well as ligand exchange between dimers. Even in noncoordinating solvents, Al-Me exchange is fast, as confirmed by proton NMR spectroscopy. For example, at −25 °C the 1H NMR spectrum of Me6Al2 comprises two signals in 1:2 ratio, as expected from the solid state structure. At 20 °C, only one signal is observed because exchange of terminal and bridging methyl groups is too fast to be resolved by NMR. The high Lewis acidity of the monomeric species is related to the size of the Al(III) center and its tendency to achieve an octet configuration.
Low valent organoaluminium compounds
The first organoaluminium compound with an Al-Al bond was reported in 1988 as (((Me3Si)2CH)2Al)2 (a dialane). They are typically prepared reduction of the dialkylaluminium chlorides by metallic potassium:[5]
- (R2AlCl)2 + 2 K → R2Al-AlR2 + 2 KCl
Another notable group of alanes are tetraalanes containing four Al(I) centres. These compounds adopt a tetrahedrane core, as illustrated by (Cp*Al)4 and ((Me3Si3C)Al)4. The cluster [Al12(i-Bu)12]2− was obtained from related investigations on the reduction of organoaluminium compounds. This dianion adopts an icosahedral structure reminiscent of dodecaborate ([B12H12]2−). Its formal oxidation state is less than one.
Preparation
From alkyl halides and aluminium
Industrially, simple aluminium alkyls of the type Al2R6 (R = Me, Et) are prepared in a two-step process beginning with the alkylation of aluminium powder:
- 2 Al + 3 CH3CH2Cl → (CH3CH2)3Al2Cl3
The reaction resembles the synthesis Grignard reagents. The product, (CH3CH2)3Al2Cl3, is called ethylaluminium sesquichloride. The term sesquichloride refers to the fact that, on average, the Cl:Al ratio is 1.5. These sesquichlorides can be converted to the triorganoaluminium derivatives by reduction:
- 2 (CH3CH2)3Al2Cl3 + 6 Na → (CH3CH2)6Al2 + 2 Al + 6 NaCl
This method is used for production of trimethylaluminium and triethylaluminium.[6]
Hydroalumination
Aluminium powder reacts directly with certain terminal alkenes in the presence of hydrogen. The process entails two steps, the first producing dialkylaluminium hydrides. Such reactions are typically conducted at elevated temperatures and require activation by trialkylaluminium reagents:
- 3 Al + 3/2 H2 + 6 CH2=CHR → [HAl(CH2CHR)2]3
For nonbulky R groups, the organoaluminium hydrides are typically trimeric. In a subsequent step, these hydrides are treated with more alkene to effect hydroalumiunation:
- 2 [HAl(CH2CHR)2]3 + 3 CH2=CHR → 3 [Al2(CH2CHR)3
Diisobutylaluminium hydride, which is dimeric, is prepared by hydride elimination from triisobutylaluminium:
- 2 i-Bu3Al → (i-Bu2AlH)2 + 2 (CH3)2C=CH2
Carboalumination
Organoaluminium compounds can react with alkenes and alkynes in carboalumination. Monoaddition is only possible when the alkene is substituted. The reaction is regioselective for 1-alkenes.[7] The so-called ZACA reaction is an example of an asymmetric carboalumination.
Laboratory preparations
Although the simple members are commercially available at low cost, many methods have been developed for their synthesis in the laboratory, including metathesis or transmetalation. Metathesis of aluminium trichloride with RLi or RMgX gives the trialkyl:
- AlCl3 + 3 BuLi → Bu3Al + 3 LiCl
- Transmetalation
- 2 Al + 3 HgPh2 → 2 AlPh3 + 3 Hg
Reactions
The high reactivity of organoaluminium compounds toward electrophiles is attributed to the charge separation between aluminum and carbon atom.
Lewis acidity
Organoaluminium compounds are hard acids and readily form adducts with bases such as pyridine, THF and tertiary amines. These adducts are tetrahedral at Al.
Electrophiles
The Al-C bond is polarized such that the carbon is highly basic. Acids react to give alkanes. For example, alcohols give alkoxides:
- AlR'3 + ROH → 1/n (R'2Al−OR)n + R'H
A wide variety of acids can be employed beyond the simple mineral acids. Amines give amido derivatives. With carbon dioxide, trialkylaluminium compounds give the dialkylaluminium carboxylate, and subsequently alkyl aluminium dicarboxylates:
- AlR3 + CO2 → R2AlO2CR
- R2AlO2CR + CO2 → RAl(O2CR)2
The conversion is reminiscent of the carbonation of Grignard reagents.[8][9][10]
Similarly, the reaction between trialkylaluminum compounds and carbon dioxide has been used to synthesise alcohols, olefins,[8] or ketones.[11]
With oxygen one obtains the corresponding alkoxides, which can be hydrolysed to the alcohols:
- AlR3 + 3/2 O2 → Al(OR)3
A structurally characterized organoaluminum peroxide is [{HC[C(Me)N-C6H5]2}Al(R)-O-O-CMe3] [R=CH(SiMe3)2].[12]
The reaction between pure trialalkylaluminum compounds and water, alcohols, phenols, amines, carbon dioxide, sulfur oxides, nitrogen oxides, halogens, and halogenated hydrocarbons can be violent.[13][14]
Alkene polymerization
Industrially, organoaluminium compounds are used as catalysts for alkene polymerization to polyolefins, for example the catalyst methylaluminoxane.
See also
| CH | He | ||||||||||||||||
| CLi | CBe | CB | CC | CN | CO | CF | Ne | ||||||||||
| CNa | CMg | CAl | CSi | CP | CS | CCl | CAr | ||||||||||
| CK | CCa | CSc | CTi | CV | CCr | CMn | CFe | CCo | CNi | CCu | CZn | CGa | CGe | CAs | CSe | CBr | CKr |
| CRb | CSr | CY | CZr | CNb | CMo | CTc | CRu | CRh | CPd | CAg | CCd | CIn | CSn | CSb | CTe | CI | CXe |
| CCs | CBa | CHf | CTa | CW | CRe | COs | CIr | CPt | CAu | CHg | CTl | CPb | CBi | CPo | CAt | Rn | |
| Fr | CRa | Rf | Db | CSg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo | |
| ↓ | |||||||||||||||||
| CLa | CCe | CPr | CNd | CPm | CSm | CEu | CGd | CTb | CDy | CHo | CEr | CTm | CYb | CLu | |||
| Ac | CTh | CPa | CU | CNp | CPu | CAm | CCm | CBk | CCf | CEs | Fm | Md | No | Lr | |||
| Core organic chemistry | Many uses in chemistry |
| Academic research, but no widespread use | Bond unknown |
References
- ↑ D. F. Shriver; P. W. Atkins (2006). Inorganic Chemistry. Oxford University Press. ISBN 978-0199264636.
- ↑ M. Witt; H. W. Roesky (2000). "Organoaluminum chemistry at the forefront of research and development" (PDF). Curr. Sci. 78 (4): 410.
- ↑ Hallwachs, W.; Schafarik, A. (1859). "Ueber die Verbindungen der Erdmetalle mit organischen Radicalen". Liebigs Ann. Chem. 109 (2): 206–209. doi:10.1002/jlac.18591090214.
- ↑ Elschenbroich, C. (2006). Organometallics (3rd ed.). Weinheim: Wiley-VCH. ISBN 978-3-527-29390-2.
- ↑ Uhl, W. (2004). "Organoelement Compounds Possessing Al---Al, Ga---Ga, In---In, and Tl---Tl Single Bonds". Adv. Organomet. Chem. 51: 53–108. doi:10.1016/S0065-3055(03)51002-4.
- ↑ Michael J. Krause, Frank Orlandi, Alfred T. Saurage and Joseph R. Zietz "Aluminum Compounds, Organic" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a01_543
- ↑ Barry M. Trost; Martin F. Semmelhack; Ian Fleming (1992). Comprehensive Organic Synthesis: Additions to and substitutions at C-C[pi]-Bonds. Pergamon. ISBN 9780080405957.
- 1 2 Yur'ev, V.P.; Kuchin, A.V.; Tolstikov, G.A. (1974). "Reaction of aluminum trialkyls with carbon dioxide". Organic And Biological Chemistry (Springer) 23 (4): 817–819. doi:10.1007/BF00923507.
- ↑ Ziegler, K. (1956). "Neue Entwicklungen der metallorganischen Synthese". Angew. Chem. 68 (23): 721. doi:10.1002/ange.19560682302.
- ↑ Zakharkin, L.I.; Gavrilenko, V.V.; Ivanov, L.L. (1967). Zh. Obshch. Khim. 377: 992. Missing or empty
|title=(help); - ↑ David W. Marshall, US patent US3168570, assigned to Continental Oil
- ↑ W. Uhl; B. Jana (2008). "A persistent alkylaluminum peroxide: Surprising stability of a molecule with strong reducing and oxidizing functions in close proximity". Chem. Eur. J. 14 (10): 3067–71. doi:10.1002/chem.200701916. PMID 18283706.
- ↑ Cameo Chemicals SDS
- ↑ Handling Chemicals Safely 1980. p. 929