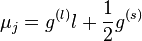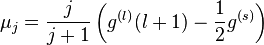Nuclear magnetic moment
The nuclear magnetic moment is the magnetic moment of an atomic nucleus and arises from the spin of the protons and neutrons. It is mainly a magnetic dipole moment; the quadrupole moment does cause some small shifts in the hyperfine structure as well. All nuclei that have nonzero spin also possess a nonzero magnetic moment and vice versa, although the connection between the two quantities is not straightforward or easy to calculate.
The nuclear magnetic moment varies from isotope to isotope of an element. For a nucleus of which the numbers of protons and of neutrons are both even in its ground state (i.e. lowest energy state), the nuclear spin and magnetic moment are both always zero. In cases with odd numbers of either or both protons and neutrons, the nucleus often has nonzero spin and magnetic moment.
Measurement methods
The methods for measuring nuclear magnetic moments can be divided into two broad groups in regard to the interaction with internal or external applied fields.[1] Generally the methods based on external fields are more accurate.
Shell model
According to the shell model, protons or neutrons tend to form pairs of opposite total angular momentum. Therefore the magnetic moment of a nucleus with even numbers of each protons and neutrons is zero, while that of a nucleus with an odd number of protons and even number of neutrons (or vice versa) will have to be that of the remaining unpaired nucleon. For a nucleus with odd numbers of each protons and neutrons, the total magnetic moment will be some combination of the magnetic moments of both of the "last", unpaired proton and neutron.
The magnetic moment is calculated through j, l and s of the unpaired nucleon, but nuclei are not in states of well defined l and s. Furthermore, for odd–odd nuclei, there are two unpaired nucleons to be considered, as in deuterium. There is consequently a value for the nuclear magnetic moment associated with each possible l and s state combination, and the actual state of the nucleus is a superposition of these. Thus the real (measured) nuclear magnetic moment is between the values associated with the "pure" states, though it may be close to one or the other (as in deuterium).
g-factors
The values of g(l) and g(s) are known as the g-factors of the nucleons.
The measured values of g(l) for the neutron and the proton are according to their electric charge. Thus, in units of nuclear magneton, g(l) = 0 for the neutron and g(l) = 1 for the proton.
The measured values of g(s) for the neutron and the proton are twice their magnetic moment (either the neutron magnetic moment or the proton magnetic moment). In nuclear magneton units, g(s) = −3.8263 for the neutron and g(s) = 5.5858 for the proton.
Calculating the magnetic moment
In the shell model, the magnetic moment of a nucleon of total angular momentum j, orbital angular momentum l and spin s, is given by
Projecting with the total angular momentum j gives
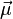 has contributions both from the orbital angular momentum and the spin, with different coefficients g(l) and g(s):
has contributions both from the orbital angular momentum and the spin, with different coefficients g(l) and g(s):
by substituting this back to the formula above and rewriting
For a single nucleon 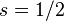 . For
. For 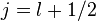 we get
we get
and for 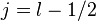
See also
- Gyromagnetic ratio
- Nuclear magneton
- Magnetic moment
- Neutron magnetic moment
- Electron magnetic moment
- Deuterium magnetic moment
- Proton spin crisis
Bibliography
- Nersesov, E.A. (1990). Fundamentals of atomic and nuclear physics. Moscow: Mir Publishers. ISBN 5-06-001249-2.
- Vonsovsky, Sergey V. (1975). Magnetism of Elemetary Particles. Mir Publishers.
- Hans Kopfermann Kernmomente and Nuclear Momenta (Akademische Verl., 1940, 1956, and Academic Press, 1958)
References
- ↑ Blyn Stoyle, Magnetic moments , p 6
External links
-
 Nuclear Structure and Decay Data - IAEA with query on Magnetic Moments
Nuclear Structure and Decay Data - IAEA with query on Magnetic Moments - magneticmoments.info/wp A blog with all recent publications on electromagnetic moments in nuclei
- Table of nuclear magnetic dipole and electric quadrupole moments, N.J. Stone
- RevModPhys Blyn Stoyle
|
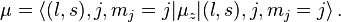
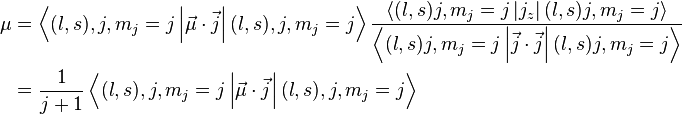
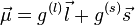
![\begin{align}
\vec{l}\cdot\vec{j} &= \frac{1}{2}\left(\vec{j} \cdot \vec{j} + \vec{l} \cdot \vec{l} - \vec{s} \cdot \vec{s}\right) \\
\vec{s}\cdot\vec{j} &= \frac{1}{2}\left(\vec{j} \cdot \vec{j} - \vec{l} \cdot \vec{l} + \vec{s} \cdot \vec{s}\right) \\
\mu &= \frac{1}{2}\left\langle(l,s),j,m_j=j\left|
g^{(l)}\frac{1}{2}\left(\vec{j} \cdot \vec{j} + \vec{l} \cdot \vec{l} - \vec{s} \cdot \vec{s}\right) +
g^{(s)}\frac{1}{2}\left(\vec{j} \cdot \vec{j} - \vec{l} \cdot \vec{l} + \vec{s} \cdot \vec{s}\right)
\right|(l,s),j,m_j=j\right\rangle \\
&= \frac{1}{j + 1}\left(g^{(l)}\frac{1}{2} \left[j(j + 1) + l(l + 1) - s(s + 1)\right] + g^{(s)}\frac{1}{2} \left[j(j + 1) - l(l + 1) + s(s + 1)\right]\right)
\end{align}](../I/m/4ed0a95c6128ba013bb0f1739badbfb5.png)