Nortriptyline
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
|
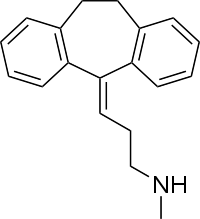
3-(10,11-Dihydro-5H-dibenzo[a,d]cyclohepten-5-ylidene)-N-methyl-1-propanamine | |
| Clinical data | |
| Trade names | Aventyl |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a682620 |
| Pregnancy category |
|
| Legal status | |
| Routes of administration | Oral |
| Pharmacokinetic data | |
| Bioavailability | High |
| Metabolism | Hepatic |
| Biological half-life | 16 and 90 hours |
| Excretion | Renal |
| Identifiers | |
| CAS Number |
72-69-5 894-71-3 (hydrochloride) |
| ATC code | N06AA10 |
| PubChem | CID 4543 |
| IUPHAR/BPS | 2404 |
| DrugBank |
DB00540 |
| ChemSpider |
4384 |
| UNII |
BL03SY4LXB |
| KEGG |
D08288 |
| ChEBI |
CHEBI:7640 |
| ChEMBL |
CHEMBL445 |
| Chemical data | |
| Formula | C19H21N |
| Molar mass | 263.38 g/mol |
| |
| |
| (verify) | |
Nortriptyline is a second-generation tricyclic antidepressant (TCA) marketed as the hydrochloride salt under the trade names Sensoval, Aventyl, Pamelor, Norpress, Allegron, Noritren and Nortrilen. It is used in the treatment of major depression and childhood nocturnal enuresis (bedwetting). In addition, it is sometimes used for chronic illnesses such as chronic fatigue syndrome, chronic pain and migraine, and labile affect in some neurological conditions.
Medical uses
Nortriptyline is FDA-approved for the treatment of major depression. In the United Kingdom, it may also be used for treating nocturnal enuresis, with courses of treatment lasting no more than three months. It is also used off-label for the treatment of panic disorder, irritable bowel syndrome, migraine prophylaxis and chronic pain or neuralgia modification, particularly temporomandibular joint disorder.[1] It can also aid in quitting smoking, with one study showing a six-month abstinence rate of 14% for subjects receiving nortriptyline compared to 3% for subjects not undergoing pharmacological treatment.[2] A controlled study suggests it reduces symptoms of ADHD.[3][4]
Neuropathic pain
Although not approved by the FDA for neuropathic pain, a large number of randomized controlled trials have demonstrated the efficacy of tricyclic antidepressants for the treatment of this condition in both depressed and non-depressed individuals. Recently, an evidence-based guideline sponsored by the International Association for the Study of Pain recommends nortriptyline as a first-line medication for neuropathic pain.[5]
Side effects
The most common side effects include dry mouth, sedation, constipation, and increased appetite, mild blurred vision, tinnitus, often euphoria and mania. An occasional side effect is a rapid or irregular heartbeat. Alcohol may exacerbate some of its side effects and should be avoided.
However, the incidence of side effects with nortriptyline is lower than with the first-generation tricyclics (e.g., imipramine (Tofranil), amitriptyline (Elavil)). For this reason it is often used in elder patients instead of other TCAs to reduce side effects and improving patient's compliance.
A study with men has found that treatment with nortriptyline is associated with higher risk of suicidal ideation compared to escitalopram.[6]
Warnings
Closer monitoring is required for those with a history of cardiovascular disease, stroke, glaucoma, or seizures, as well as those that have hyperthyroidism or are receiving thyroid medication.
Excessive consumption of alcohol in combination with nortriptyline therapy may have a potentiating effect, which may lead to the danger of increased suicidal attempts or overdosage, especially in patients with histories of emotional disturbances or suicidal ideation.
Contraindications
Nortriptyline should not be used in the acute recovery phase after myocardial infarction (e.g., heart attack). Unlike TCAs Clomipramine and Imipramine, concurrent use of Nortriptyline with MAO inhibitors is safe within recommended dosage ranges.
Overdose
The symptoms and the treatment of an overdose are largely the same as for the other tricyclic antidepressants, including serotonin syndrome and adverse cardiac effects. As tricyclic antidepressants have a relatively narrow therapeutic index, the likelihood of overdose (both accidental and intentional) is fairly high and should be considered carefully by the prescribing physician prior to patient use.
A nortriptyline overdose should always be considered a medical emergency and can result in death. In the event of a known or suspected overdose, poison control (1-800-222-1222 in the U.S.) or 911 (999 in the U.K.) should be contacted immediately. If no phone services are available the overdose victim should be brought to the nearest hospital as soon as possible.
Pharmacogenetics
Nortriptyline is metabolized in the liver by the hepatic enzyme CYP2D6, and genetic variations within the gene coding for this enzyme can affect its metabolism, leading to changes in the concentrations of the drug in the body.[7] Increased concentrations of nortriptyline may increase the risk for side effects, including anticholinergic and nervous system adverse effects, while decreased concentrations may reduce the drug's efficacy.[8][9][10]
Individuals can be categorized into different types of CYP2D6 metabolizers depending on which genetic variations they carry. These metabolizer types include poor, intermediate, extensive, and ultrarapid metabolizers. Most individuals (about 77-92%) are extensive metabolizers,[10] and have "normal" metabolism of nortriptyline. Poor and intermediate metabolizers have reduced metabolism of the drug as compared to extensive metabolizers; patients with these metabolizer types may have an increased probability of experiencing side effects. Ultrarapid metabolizers use nortriptyline much faster than extensive metabolizers; patients with this metabolizer type may have a greater chance of experiencing pharmacological failure.[8][9][10]
The Clinical Pharmacogenetics Implementation Consortium recommends avoiding nortriptyline in patients who are CYP2D6 ultrarapid or poor metabolizers, due to the risk for a lack of efficacy and side effects, respectively. A reduction in starting dose is recommended for patients who are CYP2D6 intermediate metabolizers. If use of nortriptyline is warranted, therapeutic drug monitoring is recommended to guide dose adjustments.[10] The Dutch Pharmacogenetics Working Group recommends reducing the dose of nortriptyline in CYP2D6 poor or intermediate metabolizers, and selecting an alternative drug or increasing the dose in ultrarapid metabolizers.[11]
Pharmacology
Nortriptyline is an active metabolite of amitriptyline that is demethylated in the liver. Its pharmacologic profile is as follows:[12][13]
| Receptor/Transporter Protein | Binding Affinity (Ki[nM]) | Receptor location and species |
|---|---|---|
| SERT | 16.5 | Human, cloned |
| NET | 4.37 | Human, cloned |
| DAT | 3100 | Human, cloned |
| 5-HT1A | 294 | Human, brain |
| 5-HT2A | 5 | Rat, cloned |
| 5-HT2C | 8.5 | Rat, cloned |
| 5-HT6 | 148 | Rat, cloned |
| α1 | 55 | Human, brain |
| α2 | 2030 | Human, brain |
| β | >10000 | Mammalian, brain |
| M1 | 40 | Human, cloned |
| M2 | 110 | Human, cloned |
| M3 | 50 | Human, cloned |
| M4 | 84 | Human, cloned |
| M5 | 97 | Human, cloned |
| D2 | 2570 | Human, brain |
| H1 | 15.1 | Human, cloned |
| Sigma receptor | 2000 | Guinea pig, brain |
These effects account for some therapeutic actions as well as for most side effects such as sedation, hypotension, anticholinergic effects, etc. Nortriptyline may also have a sleep-improving effect due to its affinity for 5HT2A and histaminergic receptors.[14] In the short term; however, nortriptyline may disturb sleep due to its activating effect.
Like other tricyclic antidepressants, nortriptyline also blocks sodium channels, possibly accounting in part for its analgesic action.
In one study of long-term efficacy, nortriptyline showed a higher relapse rate in comparison with phenelzine in individuals being treated for depression, possibly due to the metabolite 10-hydroxynortriptyline being produced.[15] The authors of a review noted that the nortriptyline group had more episodes prior to treatment.[15]
References
- ↑ Sweetman SC, ed. (2002). Martindale. The complete drug reference (33 ed.). Pharmaceutical Press. ISBN 0-85369-499-0.
- ↑ Prochazka AV, Weaver MJ, Keller RT, Fryer GE, Licari PA, Lofaso D (Oct 1998). "A randomized trial of nortriptyline for smoking cessation". Archives of Internal Medicine 158 (18): 2035–9. doi:10.1001/archinte.158.18.2035. PMID 9778204.
- ↑ Wilens TE, Biederman J, Geist DE, Steingard R, Spencer T (Mar 1993). "Nortriptyline in the treatment of ADHD: a chart review of 58 cases". Journal of the American Academy of Child and Adolescent Psychiatry (American Academy of Child and Adolescent Psychiatry) 32 (2): 343–9. doi:10.1097/00004583-199303000-00015. PMID 8444763.
- ↑ Prince JB, Wilens TE, Biederman J, Spencer TJ, Millstein R, Polisner DA, Bostic JQ (2000). "A controlled study of nortriptyline in children and adolescents with attention deficit hyperactivity disorder". Journal of Child and Adolescent Psychopharmacology 10 (3): 193–204. doi:10.1089/10445460050167304. PMID 11052409.
- ↑ Dworkin RH, O'Connor AB, Audette J, Baron R, Gourlay GK, Haanpää ML, Kent JL, Krane EJ, Lebel AA, Levy RM, Mackey SC, Mayer J, Miaskowski C, Raja SN, Rice AS, Schmader KE, Stacey B, Stanos S, Treede RD, Turk DC, Walco GA, Wells CD (Mar 2010). "Recommendations for the pharmacological management of neuropathic pain: an overview and literature update". Mayo Clinic Proceedings 85 (3 Suppl): S3–14. doi:10.4065/mcp.2009.0649. PMC 2844007. PMID 20194146.
- ↑ Perroud N, Uher R, Marusic A, Rietschel M, Mors O, Henigsberg N, Hauser J, Maier W, Souery D, Placentino A, Szczepankiewicz A, Jorgensen L, Strohmaier J, Zobel A, Giovannini C, Elkin A, Gunasinghe C, Gray J, Campbell D, Gupta B, Farmer AE, McGuffin P, Aitchison KJ (2009). "Suicidal ideation during treatment of depression with escitalopram and nortriptyline in genome-based therapeutic drugs for depression (GENDEP): a clinical trial". BMC Medicine 7: 60. doi:10.1186/1741-7015-7-60. PMC 2768737. PMID 19832967.
- ↑ Rudorfer MV, Potter WZ (Jun 1999). "Metabolism of tricyclic antidepressants". Cellular and Molecular Neurobiology 19 (3): 373–409. PMID 10319193.
- 1 2 Stingl JC, Brockmöller J, Viviani R (Mar 2013). "Genetic variability of drug-metabolizing enzymes: the dual impact on psychiatric therapy and regulation of brain function". Molecular Psychiatry 18 (3): 273–87. doi:10.1038/mp.2012.42. PMID 22565785.
- 1 2 Kirchheiner J, Seeringer A (Mar 2007). "Clinical implications of pharmacogenetics of cytochrome P450 drug metabolizing enzymes". Biochimica Et Biophysica Acta 1770 (3): 489–94. doi:10.1016/j.bbagen.2006.09.019. PMID 17113714.
- 1 2 3 4 Hicks JK, Swen JJ, Thorn CF, Sangkuhl K, Kharasch ED, Ellingrod VL, Skaar TC, Müller DJ, Gaedigk A, Stingl JC (May 2013). "Clinical Pharmacogenetics Implementation Consortium guideline for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants". Clinical Pharmacology and Therapeutics 93 (5): 402–8. doi:10.1038/clpt.2013.2. PMC 3689226. PMID 23486447.
- ↑ Swen JJ, Nijenhuis M, de Boer A, Grandia L, Maitland-van der Zee AH, Mulder H, Rongen GA, van Schaik RH, Schalekamp T, Touw DJ, van der Weide J, Wilffert B, Deneer VH, Guchelaar HJ (May 2011). "Pharmacogenetics: from bench to byte--an update of guidelines". Clinical Pharmacology and Therapeutics 89 (5): 662–73. doi:10.1038/clpt.2011.34. PMID 21412232.
- ↑ National Institute of Mental Health. PDSD Ki Database (Internet) [cited 2013 Oct 3]. Chapel Hill (NC): University of North Carolina. 1998-2013. Available from: http://pdsp.med.unc.edu/pdsp.php
- ↑ Brunton L, Chabner B, Knollman B. Goodman and Gilman’s The Pharmacological Basis of Therapeutics, Twelfth Edition. McGraw Hill Professional; 2010.
- ↑ Thase ME (2006). "Depression and sleep: pathophysiology and treatment". Dialogues in Clinical Neuroscience 8 (2): 217–26. PMC 3181772. PMID 16889107.
- 1 2 Kennedy SH (Mar 1997). "Continuation and maintenance treatments in major depression: the neglected role of monoamine oxidase inhibitors". Journal of Psychiatry & Neuroscience 22 (2): 127–31. PMC 1188835. PMID 9074307.
External links
- Internet Mental Health - Nortriptyline
- Drugs.com - Nortriptyline
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||