Norbadione A
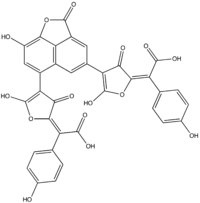 | |
| Names | |
|---|---|
| IUPAC name
(2E,2E)-2,2'-[(8-Hydroxy-2-oxo-2H-naphtho[1,8-bc]furan-4,6-diyl)bis(5-hydroxy-3-oxo-4-furanyl-2-ylidene)]bis[(4-hydroxyphenyl)acetic acid] | |
| Identifiers | |
| 90295-68-4 | |
| Jmol interactive 3D | Image |
| PubChem | 54679293 |
| |
| Properties | |
| C35H18O15 | |
| Molar mass | 678.50842 gmol−1 |
| Appearance | red needles |
| Density | 1.902 g/cm3 |
| Melting point | 300 °C (572 °F; 573 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Norbadione A is a pigment found in the bay bolete mushroom (Boletus badius). A polyphenol, norbadione A is related to a family of mushroom pigments known as pulvinic acids.[1] The molecule has also been reported as a potassium salt from the mushrooms Pisolithus tinctorius (horse dung fungus)[2] and Chalciporus piperatus.[3]
Properties
Norbadione A has seven acid-base functional groups, among which are two enolic and two carboxylic acid moieties.[4] These functional groups confer water-solubility to the molecule. It selectively complexes cesium cations (Cs+),[5] with an efficiency comparable to that of some calixarenes or crown ethers.[4] It has been investigated for its ability to provide a protective effect against the damaging effects of ionizing radiation, an effect attributed to its ability to protect DNA-related targets from irradiation.[6] Tests with cell cultures and mice show that although it has some protective effect, it is toxic to cells in higher doses.[7] A diverse array of synthetic derivatives of norbadione A has been created to explore the effect of structure on antioxidant properties and cytotoxicity.[8] A series of alkali chelators based on the structure of norbadione A has been reported.[9] The intramolecular protonation process has been determined. There is a pH-dependent Z to E isomer switch that occurs in both pulvinate moieties,[10] which yields four stereoisomeric forms (E/E, E/Z, Z/Z, Z/E). These stereoisomers may have a widely differing ability to form complexes with Cs+ in solution.[6]
Synthesis
Bourdreux and colleagues reported a total synthesis of norbadione A in 2008. The technique uses a regioselective Diels–Alder reaction and a double Suzuki-Miyaura cross-coupling.[11]
References
- ↑ Aumann DC, Clooth G, Steffan B, Steglich W. (1989). "Complexation of cesium-137 by the cap pigments of the bay boletus (Xerocomus badius)". Angewandte Chemie-International Edition in English 28 (4): 453–454. doi:10.1002/anie.198904531.
- ↑ Thompson RH. (1997). Naturally Occurring Quinones IV. Springer. p. 282. ISBN 978-0-7514-0248-3.
- ↑ Yannai S. (2013). Dictionary of Food Compounds. CRC Press. p. 1416. ISBN 978-1-4200-8351-4.
- 1 2 Korovitch A, Mulon JB, Souchon V, Leray I, Valeur B, Mallinger A, Nadal B, Le Gall T, Lion C, Ha-Duong NT, El Hage Chahine JM. (2010). "Norbadione A: kinetics and thermodynamics of cesium uptake in aqueous and alcoholic media". Journal of Physical Chemistry. B 114 (39): 12655–65. doi:10.1021/jp1060232. PMID 20831226.
- ↑ Kuad P, Schurhammer R, Maechling C, Antheaume C, Mioskowski C, Wipff G, Spiess B. (2009). "Complexation of Cs+, K+ and Na+ by norbadione A triggered by the release of a strong hydrogen bond: nature and stability of the complexes". Physical Chemistry Chemical Physics 11 (44): 10299–310. doi:10.1039/b912518c. PMID 19890513.
- 1 2 Schurhammer R, Diss R, Spiess B, Wipff G. (2007). "Conformational and Cs+ complexation properties of norbadione-A: A molecular modeling study". Physical Chemistry Chemical Physics 10: 495–505. doi:10.1039/B712836C.
- ↑ Le Roux A, Josset E, Benzina S, Nadal B, Desage-El Murr M, Heurtaux B, Taran F, Denis J-M, Le Gall T, Meunier S, Bischoff P. (2012). "Evaluation of the radioprotective potential of the polyphenol norbadione A". Letters in Drug Design & Discovery 9 (1): 48–53. doi:10.2174/157018012798192900.
- ↑ Habrant D, Poigny S, Ségur-Derai M, Brunel Y, Heurtaux B, Le Gall T, Strehle A, Saladin R, Meunier S, Mioskowski C, Wagner A. (2009). "Evaluation of antioxidant properties of monoaromatic derivatives of pulvinic acids". Journal of Medicinal Chemistry 52 (8): 2454–2464. doi:10.1021/jm801500h. PMID 19309153.
- ↑ Korovitch A, Le Roux A, Barbault F, Hémadi M, Ha-Duong N-T, Lion C, Wagner A, El Hage Chahine J-M (2013). "A new series of Cs+, K+ and Na+ chelators: Synthesis, kinetics, thermodynamics and modeling". Inorganica Chimica Acta 394: 45–57. doi:10.1016/j.ica.2012.08.009.
- ↑ Kuad P, Borkovec M, Desage-El Murr M, Le Gall T, Mioskowski C, Spiess B. (2005). "Inframolecular protonation process of norbadione A: Influence of the ionic environment and stereochemical consequences". Journal of the American Chemical Society 127 (4): 1323–1333. doi:10.1021/ja0483185. PMID 15669874.
- ↑ Bourdreux Y, Nowaczyk S, Billaud C, Mallinger A, Willis C, Murr MD, Toupet L, Lion C, Gall TL, Mioskowski C. (2008). "Total synthesis of norbadione A". Journal of Organic Chemistry 73 (1): 22–26. doi:10.1021/jo702106u. PMID 18052074.