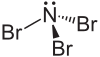Nitrogen tribromide
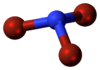
Nitrogen tribromide is a chemical compound with the formula NBr3. It is extremely explosive in its pure form, even at −100 °C, and was not isolated until 1975.[2] It is a deep-red, volatile solid, and was first prepared by the bromination of bistrimethlysilylbromamine with BrCl at −87 °C:
- (Me3Si)2NBr + 2 BrCl → NBr3 + 2 Me
3SiCl
It reacts instantly with ammonia in dichloromethane solution at −87°C to yield NBrH2.
References
- ↑ Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, FL: CRC Press, pp. 4–73, ISBN 0-8493-0594-2
- ↑ N. N. Greenwood and A. Earnshaw, "Chemistry of the Elements", 2006 Butterworth-Heinemann