Niobium oxychloride
 | |
| Names | |
|---|---|
| IUPAC name
Niobium (V) Oxychloride | |
| Other names
Niobium oxytrichloride | |
| Identifiers | |
| 13597-20-1 | |
| Properties | |
| Cl3NbO | |
| Molar mass | 215.26 g·mol−1 |
| Appearance | white crystals |
| Melting point | sublimes above 200 °C |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Niobium oxychloride is the inorganic compound with the formula NbOCl3. It is a white, crystalline, diamagnetic solid. It is often found as an impurity in samples of niobium pentachloride, a common reagent in niobium chemistry.
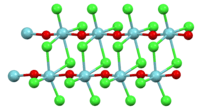
Structure
In the solid state the coordination sphere for niobium is a distorted octahedron. The Nb-O bonds and Nb-Cl bonds are unequal. This structure can be described as planar Nb2Cl6 core connected by O-Nb-O bridges. In this way, the compound is best described as a polymer, consisting of a double stranded chain.[1][2]
In the gas phase above 320 °C the Raman spectrum is consistent with a pyramidal monomer containing a niobium oxygen double bond.[3]

Synthesis
Niobium oxychloride is prepared by treating the pentachloride with oxygen:[4]
- NbCl5 + 1/2 O2 → NbOCl3 + Cl2
This reaction is conducted at about 200 °C. NbOCl3 also forms as a major side-product in the reaction of niobium pentoxide with various chlorinating agents such as carbon tetrachloride and thionyl chloride.[4][5]
- 2 Nb2O5 + 6 CCl4 → 4 NbOCl3 + 6 COCl2
References
- ↑ Ströbele, Markus; Meyer, Hans-Jürgen (2002). "Neubestimmung der Kristallstruktur von NbOCl3". Zeitschrift für anorganische und allgemeine Chemie 628: 488–491. doi:10.1002/1521-3749(200202)628:2<488::AID-ZAAC488>3.0.CO;2-B.
- ↑ Beck, Johannes; Bordinhão, Jairo (2005). "Polar [NbOCl3]2n and [NbOX4−]n (X = Cl, Br) Chains in the Structures of NbOCl3 and the Thallium-Halogenooxoniobates Tl[NbOCl4] and Tl[NbOBr4] - Synthesis, Crystal Structures and Optical Second Harmonic Generation". Zeitschrift für anorganische und allgemeine Chemie 631 (6–7): 1261–1266. doi:10.1002/zaac.200500041.
- ↑ Greenwood, N.N. (January 1, 1970). "Chapter 5: Vibrational Spectra". Spectroscopic Properties of Inorganic and Organometallic Compounds. Royal Society of Chemistry. p. 196. ISBN 9780851860237.
- 1 2 "Niobium Oxytrichloride" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 2. pp. 1307.
- ↑ Jena, P. K.; Brocchi, E. A.; Garcia, R. I. (1997). "Kinetics of chlorination of niobium pentoxide by carbon tetrachloride". Metallurgical and Materials Transactions B 28: 39–45. doi:10.1007/s11663-997-0125-0..
| ||||||