Nintedanib
 Nintedanib | |
| Systematic (IUPAC) name | |
|---|---|
|
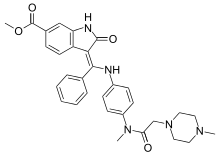
Methyl (3Z)-3-{[(4-{methyl[(4-methylpiperazin-1-yl)acetyl]amino}phenyl)amino](phenyl)methylidene}-2-oxo-2,3-dihydro-1H-indole-6-carboxylate | |
| Clinical data | |
| Trade names | Vargatef, Ofev |
| AHFS/Drugs.com | Consumer Drug Information |
| Pregnancy category |
|
| Legal status |
|
| Routes of administration | Oral and intravenous |
| Pharmacokinetic data | |
| Bioavailability | 4.7% |
| Protein binding | 97.8% |
| Metabolism | Esterases, glucuronidation |
| Biological half-life | 10–15 hrs |
| Excretion | 93% via faeces |
| Identifiers | |
| CAS Number |
656247-17-5 |
| ATC code | L01XE31 |
| PubChem | CID 9809715 |
| IUPHAR/BPS | 5936 |
| ChemSpider |
7985471 |
| UNII |
G6HRD2P839 |
| ChEBI |
CHEBI:85164 |
| ChEMBL | CHEMBL502835 |
| Synonyms | BIBF 1120 |
| Chemical data | |
| Formula | C31H33N5O4 |
| Molar mass | 539.6248 g/mol |
| |
| |
| | |
Nintedanib, marketed under the brand names Ofev and Vargatef, is a medication used for the treatment of idiopathic pulmonary fibrosis (IPF) and along with other medications for some types of non-small-cell lung cancer.
Common side effects include abdominal pain, vomiting, and diarrhea.[1] It is a small molecule tyrosine-kinase inhibitor, targeting vascular endothelial growth factor receptor (VEGFR), fibroblast growth factor receptor (FGFR) and platelet derived growth factor receptor (PDGFR).
It was developed by Boehringer Ingelheim. At an assumed cost of 39,300 pounds per year it does not appear to be cost effective for IPF in the United Kingdom.[2]
Medical uses
Idiopathic pulmonary fibrosis
Nintedanib is used for the treatment of idiopathic pulmonary fibrosis (IPF).[3] It has been shown to slow down decrease in forced vital capacity,[4][5] and it also improves people's quality of life.[6]
Lung cancer
It is also used in combination with docetaxel as a second line treatment for adult patients with locally advanced, metastatic, or locally recurring NSCLC of adenocarcinoma histology.[7] It is unclear how this combination compares to other second line agents as the comparisons have not been done as of 2014.[7]
Contraindications
Nintedanib has not been tested in patients with moderate to severe impairment of liver function. Given that the drug is metabolised in the liver, it may not be safe in such patients.[8]
Adverse effects
Preclinical studies have shown that nintedanib binds in a highly selective manner to the ATP binding pocket of its three target receptor families, without binding to similarly shaped ATP domains in other proteins, which reduces the potential for undesirable side effects.[9]
The most common side effects observed with nintedanib were reversible elevation in liver enzymes (10 to 28% of patients) and gastrointestinal disturbance (up to 50%). Side effects observed with nintedanib were worse with the higher dose, for this reason subsequent trials have used the equally clinically effective lower dose.[9][10][11][12][13][14][15][16][17]
Nintedanib inhibits the growth and reshaping of blood vessels which is also an essential process in normal wound healing and tissue repair. Therefore, a theoretical side effect of nintedanib is reduced wound healing however, unlike other anti-angiogenic agents, this side effect has not been observed in patients receiving nintedanib.
Interactions
Nintedanib is a substrate of the transporter P-glycoprotein (P-gp) which moves the absorbed substance back in to the gut's lumen. The P-gp inhibitor ketoconazole is known to increase blood plasma levels of nintedanib by a factor of 1.8; other inhibitors such as erythromycin or ciclosporin are expected to have a similar effect. On the other hand, the P-gp inductor rifampicin has been shown to cut nintedanib plasma levels in half; other inductors such as carbamazepine, phenytoin or St. John's Wort probably lower plasma levels as well.[8]
Pharmacology
Mechanism of action
Idiopathic pulmonary fibrosis
Nintedanib targets growth factor receptors, which have been shown to be involved in the mechanisms by which pulmonary fibrosis occurs. Most importantly nintedanib inhibits platelet-derived growth factor receptor (PDGFR), fibroblast growth factor receptor (FGFR) and vascular endothelial growth factor receptor (VEGFR). It is believed that nintedanib reduces disease progression in IPF and slows the decline in lung function by blocking the signalling pathways that are involved in fibrotic processes.[18][19]
Lung cancer
Nintedanib is an indolinone-derived drug that inhibits the process of blood vessel formation (angiogenesis). Angiogenesis inhibitors stop the formation and reshaping of blood vessels in and around tumours, which reduces the tumour's blood supply, starving tumour cells of oxygen and nutrients leading to cell death and tumour shrinkage. Unlike conventional anti-cancer chemotherapy which has a direct cell killing effect on cancer cells, angiogenesis inhibitors starve the tumour cells of oxygen and nutrients which results in tumour cell death. One of the advantages of this method of anti-cancer therapy is that it is more specific than conventional chemotherapy agents, therefore results in fewer and less severe side effects than conventional chemotherapy.
Angiogenesis is a process that is essential for the growth and spread of all solid tumours, blocking it prevents the tumour from growing and may result in tumour shrinkage as well as a reduction in the spread of the cancer to other parts of the body. Nintedanib exerts its anti-cancer effect by binding to and blocking the activation of cell receptors involved in blood vessel formation and reshaping (i.e. VEGFR 1-3, FGFR 1-3 and PDGFRα and β). Inhibition of these receptors in the cells that make up blood vessels (endothelial cells, smooth muscle cells and pericytes) by nintedanib leads to programmed cell death, destruction of tumor blood vessels and a reduction in blood flow to the tumour. Reduced tumour blood flow inhibits tumor cell proliferation and migration hence slowing the growth and spread of the cancer.[10]
Pharmacokinetics

Only a small percentage of orally taken nintedanib is absorbed in the gut, partially due to transport proteins (such as P-glycoprotein) moving the substance back into the lumen. Combined with a high first-pass effect, this results in an oral bioavailability of about 4.7%.[8]
Nintedanib is mainly inactivated by esterases that cleave the methyl ester, resulting in the free carboxylic acid (BIBF 1202), which is then glucuronidated by enzymes called uridinediphosphate-glucuronosyltransferases (UGTs) and excreted mostly via the bile and faeces. No relevant cytochrome P450 mediated metabolism has been observed.[8]
Physical and chemical properties

The drug is used in form of its salt with ethanesulfonic acid. This salt, nintedanib esliate, is a yellow, crystalline solid that melts at 244 °C (471 °F) to 251 °C (484 °F). It is very badly soluble in water, and somewhat better soluble in dimethyl sulfoxide (DMSO) at 25 g/l.[20]
History
Idiopathic pulmonary fibrosis
Nintedanib was approved for idiopathic pulmonary fibrosis on 15 Oct 2014 by the Food and Drug Administration,[21] and received a positive opinion from the European Medicines Agency on 20 November 2014, being approved in the EU in January 2015.[22] It is also approved in Canada, Japan, Switzerland, and other countries.
Lung cancer
Nintedanib was approved for combination therapy of non-small-cell lung cancer in the European Union in 2014,[8][23] and is possibly approved for this indication in other parts of the world.
Society and culture
A review, which assumed the price of nintebanib was 39,300 pounds a year found that it was not cost effective.[2]
Boehringer is using the brand name Ofev for marketing nintedanib for idiopathic pulmonary fibrosis and Vargatef for marking the medication for lung cancer.[24]
Research
Nintedanib is being tested in several phase I to III clinical trials for cancer. Angiogenesis inhibitors such as nintedanib may be effective in a range of solid tumour types including lung, ovarian, metastatic bowel, liver and brain cancer.
Current phase II trials are investigating the effect of nintedanib in patients with metastatic bowel cancer, liver cancer and the brain tumour glioblastoma multiforme.[25]
Phase III trials are investigating the use of nintedanib in combination with carboplatin and paclitaxel as a first line treatment for patients with ovarian cancer.[26]
References
- ↑ "Nintedanib Side Effects". Retrieved 24 October 2015.
- 1 2 Loveman, E; Copley, VR; Colquitt, JL; Scott, DA; Clegg, AJ; Jones, J; O'Reilly, KM; Singh, S; Bausewein, C; Wells, A (19 November 2014). "The effectiveness and cost-effectiveness of treatments for idiopathic pulmonary fibrosis: systematic review, network meta-analysis and health economic evaluation". BMC pharmacology & toxicology 15: 63. doi:10.1186/2050-6511-15-63. PMC 4247619. PMID 25410822.
- ↑ "Nintedanib". drugs.com. Retrieved 12 February 2015.
- ↑ Ahluwalia, N; Shea, BS; Tager, AM (15 October 2014). "New therapeutic targets in idiopathic pulmonary fibrosis. Aiming to rein in runaway wound-healing responses.". American Journal of Respiratory and Critical Care Medicine 190 (8): 867–78. doi:10.1164/rccm.201403-0509pp. PMID 25090037.
- ↑ Mazzei, ME; Richeldi, L; Collard, HR (June 2015). "Nintedanib in the treatment of idiopathic pulmonary fibrosis.". Therapeutic advances in respiratory disease 9 (3): 121–9. doi:10.1177/1753465815579365. PMID 25862013.
- ↑ Dimitroulis, IA (September 2014). "Nintedanib: a novel therapeutic approach for idiopathic pulmonary fibrosis". Respiratory care 59 (9): 1450–5. doi:10.4187/respcare.03023. PMID 24782550.
- 1 2 Popat, S; Mellemgaard, A; Fahrbach, K; Martin, A; Rizzo, M; Kaiser, R; Griebsch, I; Reck, M (5 December 2014). "Nintedanib plus docetaxel as second-line therapy in patients with non-small-cell lung cancer: a network meta-analysis". Future oncology (London, England) 11 (3): 1–12. doi:10.2217/fon.14.290. PMID 25478720.
- 1 2 3 4 5 Haberfeld, H (ed.). Austria-Codex (in German) (2014/2015 ed.). Vienna: Österreichischer Apothekerverlag.
- 1 2 Hilberg, F.; U. Tontsch-Grunt, F. Colbatzky, A. Heckel, R. Lotz, J.C.A. van Meel, G.J. Roth (2004). "BIBF1120 a novel, small molecule triple angiokinase inhibitor: profiling as a clinical candidate for cancer therapy". European Journal of Cancer Supplements 2 (50). Cite uses deprecated parameter
|coauthors=(help) - 1 2 Hilberg, F.; G. J. Roth, M. Krssak, S. Kautschitsch, W. Sommergruber, U. Tontsch-Grunt, P. Garin-Chesa, G. Bader, A. Zoephel, J. Quant, A. Heckel, W. J. Rettig (2008). "BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy". Cancer Res 68 (12): 4774–82. doi:10.1158/0008-5472.CAN-07-6307. ISSN 1538-7445. PMID 18559524. Cite uses deprecated parameter
|coauthors=(help) - ↑ Reck, M.; R. Kaiser; C. Eschbach; M. Stefanic; J. Love; U. Gatzemeier; P. Stopfer; J. von Pawel (2011). "A phase II double-blind study to investigate efficacy and safety of two doses of the triple angiokinase inhibitor BIBF 1120 in patients with relapsed advanced non-small-cell lung cancer". Ann Oncol. ISSN 1569-8041.
- ↑ Okamoto, I.; H. Kaneda, T. Satoh, W. Okamoto, M. Miyazaki, R. Morinaga, S. Ueda, M. Terashima, A. Tsuya, A. Sarashina, K. Konishi, T. Arao, K. Nishio, R. Kaiser, K. Nakagawa (2010). "Phase I safety, pharmacokinetic, and biomarker study of BIBF 1120, an oral triple tyrosine kinase inhibitor in patients with advanced solid tumors". Mol Cancer Ther 9 (10): 2825–33. doi:10.1158/1535-7163.MCT-10-0379. ISSN 1538-8514. PMID 20688946. Cite uses deprecated parameter
|coauthors=(help) - ↑ Mross, K.; M. Stefanic, D. Gmehling, A. Frost, F. Baas, C. Unger, R. Strecker, J. Henning, B. Gaschler-Markefski, P. Stopfer, L. de Rossi, R. Kaiser (2010). "Phase I study of the angiogenesis inhibitor BIBF 1120 in patients with advanced solid tumors". Clin Cancer Res 16 (1): 311–9. doi:10.1158/1078-0432.CCR-09-0694. ISSN 1078-0432. PMID 20028771. Cite uses deprecated parameter
|coauthors=(help) - ↑ Ledermann, J.A. (2009). "A randomised phase II placebo-controlled trial using maintenance therapy to evaluate the vascular targeting agent BIBF 1120 following treatment of relapsed ovarian cancer (OC)". J Clin Oncol 27 (15s): (suppl; abstr 5501).
- ↑ Kropff, M.; J. Kienast; G. Bisping; W. E. Berdel; B. Gaschler-Markefski; P. Stopfer; M. Stefanic; G. Munzert (2009). "An open-label dose-escalation study of BIBF 1120 in patients with relapsed or refractory multiple myeloma". Anticancer Res 29 (10): 4233–8. ISSN 1791-7530. PMID 19846979.
- ↑ Ellis, P. M.; R. Kaiser; Y. Zhao; P. Stopfer; S. Gyorffy; N. Hanna (2010). "Phase I open-label study of continuous treatment with BIBF 1120, a triple angiokinase inhibitor, and pemetrexed in pretreated non-small cell lung cancer patients". Clin Cancer Res 16 (10): 2881–9. doi:10.1158/1078-0432.CCR-09-2944. ISSN 1078-0432. PMID 20460487.
- ↑ du Bois, A.; J. Huober; P. Stopfer; J. Pfisterer; P. Wimberger; S. Loibl; V. L. Reichardt; P. Harter (2010). "A phase I open-label dose-escalation study of oral BIBF 1120 combined with standard paclitaxel and carboplatin in patients with advanced gynecological malignancies". Ann Oncol 21 (2): 370–5. doi:10.1093/annonc/mdp506. ISSN 1569-8041. PMID 19889612.
- ↑ Selman, M; King, T. E.; Pardo, A; American Thoracic, Society; European Respiratory, Society; American College of Chest Physicians (2001). "Idiopathic pulmonary fibrosis: Prevailing and evolving hypotheses about its pathogenesis and implications for therapy". Annals of internal medicine 134 (2): 136–51. doi:10.7326/0003-4819-134-2-200101160-00015. PMID 11177318.
- ↑ Wollin, L; Maillet, I; Quesniaux, V; Holweg, A; Ryffel, B (2014). "Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis". Journal of Pharmacology and Experimental Therapeutics 349 (2): 209–20. doi:10.1124/jpet.113.208223. PMID 24556663.
- ↑ Sicherheitsdatenblatt [Safety data sheet] Nintedanibesilat (German)
- ↑ "FDA Approves Ofev". drugs.com. Retrieved 12 February 2015.
- ↑ "OFEV (nintedanib*) approved in the EU for the treatment of IPF". Boehringer Ingelheim Press Release Archive. 19 January 2015. Retrieved 13 May 2015.
- ↑ "Vargatef (nintedanib*) approved in the EU for lung cancer patients with advanced adenocarcinoma after first-line chemotherapy". Boehringer Ingelheim Press Release Archive. 27 November 2014. Retrieved 13 May 2015.
- ↑ "Boehringer's Ofev approved by FDA for rare lung disease". Oct 17, 2014. Retrieved 24 October 2015.
- ↑ ClinicalTrials.gov: BIBF 1120
- ↑ Clinical trial number NCT01015118 for "LUME-Ovar 1: Nintedanib (BIBF 1120) or Placebo in Combination With Paclitaxel and Carboplatin in First Line Treatment of Ovarian Cancer" at ClinicalTrials.gov
External links
| ||||||||||||||||||||||||||||||