Cell-cycle analysis
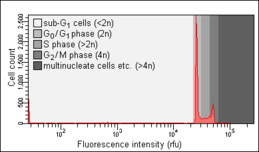
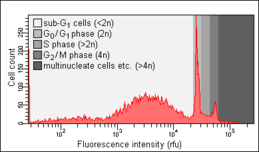
Cell-cycle analysis is a method in cell biology that employs flow cytometry to distinguish cells in different phases of the cell cycle. Before analysis, the cells are permeabilised and treated with a fluorescent dye that stains DNA quantitatively, usually propidium iodide (PI). The fluorescence intensity of the stained cells at certain wavelengths will therefore correlate with the amount of DNA they contain. As the DNA content of cells duplicates during the S phase of the cell cycle, the relative amount of cells in the G0 phase and G1 phase (before S phase), in the S phase, and in the G2 phase and M phase (after S phase) can be determined, as the fluorescence of cells in the G2/M phase will be twice as high as that of cells in the G0/G1 phase.
Cell-cycle anomalies can be symptoms for various kinds of cell damage, for example DNA damage, which cause the cell to interrupt the cell cycle at certain checkpoints to prevent transformation into a cancer cell (carcinogenesis). Other possible reasons for anomalies include lack of nutrients, for example after serum deprivation.
Cell cycle analysis was first described in 1969 at Los Alamos Scientific Laboratory by a group from the University of California,[1] using the Feulgen staining technique. The first protocol for cell-cycle analysis using propidium iodide staining was presented in 1975 by Awtar Krishan from Harvard Medical School[2] and is still widely cited today.
Experimental procedure
The first step in preparing cells for cell-cycle analysis is permeabilisation of the cells' plasma membranes. This is usually done by incubating them in a buffer solution containing a detergent[3] such as Triton X-100 or NP-40, or by fixating them in ethanol. Most fluorescent DNA dyes are not membrane permeable, that is, unable to pass through an intact cell membrane. Permeabilisation is therefore crucial for the success of the next step, the staining of the cells.
Prior to or during the staining step, the cells will usually be treated with RNase A to remove RNAs from the cells. This is important because dyes that stain DNA will also stain RNA, thus creating artefacts that would distort the results.
Aside from propidium iodide, quantifiable dyes that are frequently used include (but are not limited to) DRAQ5, 7-Aminoactinomycin D, DAPI and Hoechst 33342.
When the cells pass through the flow cytometer's laser, a fluorescence pulse is generated that correlates with the amount of dye associated with the DNA and thus with the total amount of DNA in the cell.
Doublet discrimination
Because cells and especially fixated cells tend to stick together, cell aggregates have to be excluded from analysis through a process called doublet discrimination. This is important because a doublet of two cells in the G0/G1 phase has the same total amount of DNA and thus the same fluorescence intensity as a single cell in the G2/M phase.[4] G0/G1 doublets would therefore create false positive results for G2/M cells.
Related Methods
Nicoletti assay
The Nicoletti assay, named after its inventor, the Italian physician Ildo Nicoletti, is a modified form of cell cycle analysis. It is used to detect and measure apoptosis, a form of programmed cell death, by analysing cells with a DNA content less than 2n ("sub-G1 cells"). Such cells are usually the result of apoptotic DNA fragmentation: during apoptosis, the DNA is degraded by cellular endonucleases. Therefore, nuclei of apoptotic cells contain less DNA than nuclei of healthy G0/G1 cells, resulting in a sub-G0/G1 peak in the fluorescence histogram that can be used to determine the relative amount of apoptotic cells in a sample.
This method was developed and first described in 1991 by Nicoletti and co-workers at Perugia University School of Medicine.[5] An optimised protocol developed by two of the authors of the original publication was published in 2006.[6]
| Nicoletti assay with healthy (left) and apoptotic (middle and right) cells | ||||||
|---|---|---|---|---|---|---|
|
References
- ↑ Van Dilla MA, Trujillo TT, Mullaney PF, Coulter JR (14 March 1969). "Cell Microfluorometry: A Method for Rapid Fluorescence Measurement". Science 163 (3872): 1213–1214. doi:10.1126/science.163.3872.1213. PMID 5812751.
- ↑ Krishan A. (July 1975). "Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining". The Journal of Cell Biology 66 (1): 188–193. doi:10.1083/jcb.66.1.188. PMC 2109516. PMID 49354.
- ↑ Vindeløv LL, Christensen IJ, Nissen NI (March 1983). "A detergent-trypsin method for the preparation of nuclei for flow cytometric DNA analysis". Cytometry 3 (5): 323–327. doi:10.1002/cyto.990030503. PMID 6188586.
- ↑ Wersto RP, Chrest FJ, Leary JF, Morris C, Stetler-Stevenson MA, Gabrielson E (15 October 2001). "Doublet discrimination in DNA cell-cycle analysis". Cytometry 46 (5): 296–306. doi:10.1002/cyto.1171. PMID 11746105.
- ↑ Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C (3 June 1991). "A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry". Journal of Immunological Methods 139 (2): 271–279. doi:10.1016/0022-1759(91)90198-O. PMID 1710634.
- ↑ Riccardi C, Nicoletti I (9 November 2006). "Analysis of apoptosis by propidium iodide staining and flow cytometry". Nature Protocols 1 (3): 1458–1461. doi:10.1038/nprot.2006.238. PMID 17406435.
Further reading
- "Cell Cycle Basics" (PDF, 0.1 MB). University College London. Retrieved 2010-05-20.
- Rabinovitch, Peter. "Introduction to Cell Cycle Analysis" (PDF, 0.5 MB). Phoenix Flow Systems, Inc. Retrieved 2010-05-20.
- "Label-free Cell Cycle Analysis". Phase Holographic Imaging AB. Retrieved 2013-07-28.