Vinorelbine
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
|
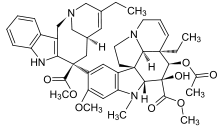
4-(acetyloxy)- 6,7-didehydro- 15-((2R,6R,8S)-4-ethyl- 1,3,6,7,8,9-hexahydro- 8-(methoxycarbonyl)- 2,6-methano- 2H-azecino(4,3-b)indol-8-yl)- 3-hydroxy- 16-methoxy- 1-methyl- methyl ester, | |
| Clinical data | |
| Trade names | Navelbine |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a695013 |
| Pregnancy category | |
| Legal status | |
| Routes of administration | intravenous, oral |
| Pharmacokinetic data | |
| Bioavailability | 43 ± 14% (oral)[1] |
| Protein binding | 79 to 91% |
| Metabolism | Hepatic (CYP3A4-mediated) |
| Biological half-life | 27.7 to 43.6 hours |
| Excretion | Fecal (46%) and renal (18%) |
| Identifiers | |
| CAS Number |
71486-22-1 |
| ATC code | L01CA04 |
| PubChem | CID 5311497 |
| DrugBank |
DB00361 |
| ChemSpider |
4470974 |
| UNII |
Q6C979R91Y |
| KEGG |
D08680 |
| ChEMBL |
CHEMBL607994 |
| Chemical data | |
| Formula | C45H54N4O8 |
| Molar mass | 778.932 g/mol |
| |
| |
| | |
Vinorelbine (NVB), sold under the trade name Navelbine, is a chemotherapy medication that is used to treatment a number of types of cancer, including breast cancer and non-small cell lung cancer.[2]
It is on the World Health Organization's List of Essential Medicines, the most important medication needed in a basic health system.[3] The wholesale price as of 2014 is between 8.60 and 42.80 USD a vial.[4][5]
Medical uses
Vinorelbine is approved for the treatment of non-small-cell lung cancer. It is used off-label for other cancers such as metastatic breast cancer. It is also active in rhabdomyosarcoma.[6]
Side effects
Vinorelbine has a number of side-effects that can limit its use:
Chemotherapy-induced peripheral neuropathy (a progressive, enduring and often irreversible tingling numbness, intense pain, and hypersensitivity to cold, beginning in the hands and feet and sometimes involving the arms and legs[7]), lowered resistance to infection, bruising or bleeding, anaemia, constipation, diarrhea, nausea, tiredness and a general feeling of weakness (asthenia), inflammation of the vein into which it was injected (phlebitis). Seldom severe hyponatremia is seen.
Less common effects are hair loss and allergic reaction.
Pharmacology
The antitumor activity is due to inhibition of mitosis through interaction with tubulin.[8] Vinorelbine is the first 5´NOR semi-synthetic vinca alkaloid. It is obtained by semi-synthesis from alkaloids extracted from the rosy periwinkle, Catharanthus roseus. It is marketed in India by Abbott Healthcare under the brand name Navelbine.
History
Vinorelbine was invented by the pharmacist Pierre Potier and his team from the CNRS in France in the 1980s and was licensed to the oncology department of the Pierre Fabre Group. It is a semi-synthetic vinca alkaloid. The drug was approved in France in 1989 under the brand name Navelbine for the treatment of non-small cell lung cancer. It gained approval to treat metastatic breast cancer in 1991. Vinorelbine received approval by the United States Food and Drug Administration (FDA) in December 1994 sponsored by Burroughs Wellcome Company. Pierre Fabre Group now markets Navelbine in the U.S., where the drug went generic in February 2003.
In most European countries, vinorelbine is approved to treat non-small cell lung cancer and breast cancer. In the United States it is approved only for non-small cell lung cancer.
Oral formulation
An oral formulation has been marketed and registered in most European countries. It has similar efficacy as the intravenous formulation, but it avoids venous toxicities of an infusion and is easier to take. The oral form is not approved in the United States or Australia.
References
- ↑ Marty M, Fumoleau P, Adenis A, Rousseau Y, Merrouche Y, Robinet G, Senac I, Puozzo C (2001). "Oral vinorelbine pharmacokinetics and absolute bioavailability study in patients with solid tumors". Ann Oncol 12 (11): 1643–9. doi:10.1023/A:1013180903805. PMID 11822766.
- ↑ "Navelbine". The American Society of Health-System Pharmacists. Retrieved Nov 28, 2015.
- ↑ "19th WHO Model List of Essential Medicines (April 2015)" (PDF). WHO. April 2015. Retrieved May 10, 2015.
- ↑ "Vinorelbine". International Drug Price Indicator Guide. Retrieved 28 November 2015.
- ↑ "Vinorelbine". International Drug Price Indicator Guide. Retrieved 28 November 2015.
- ↑ Casanova, M; Ferrari, A; Spreafico, F; Terenziani, M; Massimino, M; Luksch, R; Cefalo, G; Polastri, D; et al. (2002). "Vinorelbine in previously treated advanced childhood sarcomas: Evidence of activity in rhabdomyosarcoma". Cancer 94 (12): 3263–8. doi:10.1002/cncr.10600. PMID 12115359.
- ↑ del Pino BM. Chemotherapy-induced Peripheral Neuropathy. NCI Cancer Bulletin. Feb 23, 2010;7(4):6.
- ↑ Jordan, M.A.; Wilson, L. (2004). "Microtubules as a target for anticancer drugs.". Nature Reviews. Cancer 4 (4): 253–65. doi:10.1038/nrc1317. PMID 15057285.
| ||||||||||||||||||||||||||||||||||||||