Anthraquinones
For the parent molecule 9,10-antraquinone, see here
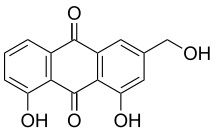
Anthraquinones (also known as anthraquinonoids) are a class of naturally occurring phenolic compounds based on the 9,10-anthraquinone skeleton.
Biosynthesis
A type II polyketide synthase is responsible for anthraquinone biosynthesis in the bacterium Photorhabdus luminescens.[1] Chorismate, formed by isochorismate synthase in the shikimate pathway, is an important precursor of anthraquinones in Morinda citrifolia. [2]
Test for anthraquinones in natural extracts
0.5g of plant extract is shaken with 10 ml of benzene and filtered. 5ml of 10% ammonia is added to the filtrate. The mixture is shaken and the presence of pink, red or violet colour indicates the presence of anthraquinones.[3]
Derivatives
Hypericin and fagopyrin are naphthodianthrones, anthraquinone-derivatives.
Applications
Many quinones are stable in multiple redox states; as such they can absorb electrons when appropriate counterions are available to maintain the bulk neutrality of the material. As such, 2,6-dihydroanthraquinone can be used as a negative electrolyte reservoir in an alkaline flow battery (opposite ferrocyanide) to store electrons when a current is applied at an ion-selective membrane.[4]
Anthraquinones are also used as laxatives such as in the drug Senna glycoside.
References
- ↑ Brachmann, AO; Joyce, SA; Jenke-Kodama, H; Schwär, G; Clarke, DJ; Bode, HB (2007). "A type II polyketide synthase is responsible for anthraquinone biosynthesis in Photorhabdus luminescens". Chembiochem : a European journal of chemical biology 8 (14): 1721–8. doi:10.1002/cbic.200700300. PMID 17722122.
- ↑ Stalman, M; Koskamp, AM; Luderer, R; Vernooy, JH; Wind, JC; Wullems, GJ; Croes, AF (2003). "Regulation of anthraquinone biosynthesis in cell cultures of Morinda citrifolia". Journal of plant physiology 160 (6): 607–14. doi:10.1078/0176-1617-00773. PMID 12872482.
- ↑ Akinjogunla OJ, Yah CS, Eghafona NO and Ogbemudia FO (2010). "Antibacterial activity of leave extracts of Nymphaea lotus (Nymphaeaceae) on Methicillin resistant Staphylococcus aureus (MRSA) and Vancomycin resistant Staphylococcus aureus (VRSA) isolated from clinical samples". Annals of Biological Research 1 (2): 174–184.
- ↑ Kaixiang Lin; et al. "Alkaline quinone flow battery". Science 349 (6255): 1529–1530. doi:10.1126/science.aab3033.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||