Naftidrofuryl
 | |
| Systematic (IUPAC) name | |
|---|---|
|
(RS)-2-(diethylamino)ethyl 3-(1-naphthyl)-2-(tetrahydrofuran-2-ylmethyl)propanoate | |
| Clinical data | |
| Trade names | Praxilene |
| AHFS/Drugs.com | International Drug Names |
| Legal status |
|
| Routes of administration | Oral |
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Biological half-life | 1 - 3.5 hours |
| Identifiers | |
| CAS Number |
31329-57-4 |
| ATC code | C04AX21 |
| PubChem | CID 4417 |
| ChemSpider |
4264 |
| UNII |
42H8PQ0NMJ |
| ChEMBL | CHEMBL1620794 |
| Chemical data | |
| Formula | C24H33NO3 |
| Molar mass | 383.524 g/mol |
| |
| |
| (verify) | |
Naftidrofuryl (INN, also known as nafronyl or as the oxalate salt naftidrofuryl oxalate or nafronyl oxalate) is a drug used in the management of peripheral and cerebral vascular disorders as a vasodilator. It is also claimed to enhance cellular oxidative capacity, and may also be a 5-HT2 receptor antagonist.[1] It is also licensed for the treatment of intermittent claudication due to peripheral arterial disease.
Naftidrofuryl is marketed under the trade names Artocoron; Azunaftil; Di-Actane; Dusodril; Enelbin; Frilix; Gevatran; Iridus; Iridux; Luctor; Nafti; Naftoling; Naftodril; Nafoxal; Praxilene; Sodipryl retard; Vascuprax.
Historically, it has been used to treat sudden idiopathic hearing loss and acute tinnitus.[2]
Naftidrofuryl may be effective for relieving the pain of muscle cramps.[3]
Synthesis
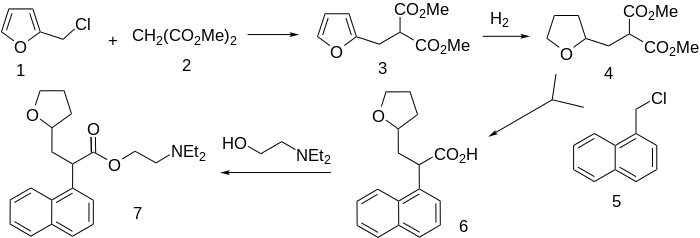
Adverse Effects
Naftidrofuryl has been associated with nausea, abdominal pain and rash. Rarely, hepatitis and liver failure have been reported.[1]
References
- 1 2 Brayfield, A, ed. (14 January 2014). "Naftidrofuryl Oxalate". Martindale: The Complete Drug Reference. Pharmaceutical Press. Retrieved 6 August 2014.
- ↑ http://www.der-arzneimittelbrief.de/Jahrgang2004/Ausgabe12Seite89.htm
- ↑ Katzberg HD, Khan AH, So YT (February 2010). "Assessment: Symptomatic treatment for muscle cramps (an evidence-based review): Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology". Neurology 74 (8): 691–6. doi:10.1212/WNL.0b013e3181d0ccca. PMID 20177124.
- ↑ Szarvasi et al., Compt. Rend. 260, 3095 (1965).
- ↑ Bull. Soc. Chim. Fr. 1966, 1838.
| ||||||||||||||||||||||||||