Nuclear quadrupole resonance
Nuclear quadrupole resonance spectroscopy or NQR is a chemical analysis technique related to nuclear magnetic resonance (NMR).[1] Unlike NMR, NQR transitions of nuclei can be detected in the absence of a magnetic field, and for this reason NQR spectroscopy is referred to as "zero Field NMR." The NQR resonance is mediated by the interaction of the electric field gradient (EFG) with the quadrupole moment of the nuclear charge distribution. Because the EFG at the location of a nucleus in a given substance is determined primarily by the valence electrons involved in the particular bond with other nearby nuclei, the NQR frequency at which transitions occur is unique for a given substance. A particular NQR frequency in a compound or crystal is proportional to the product of the nuclear quadrupole moment, a property of the nucleus, and the EFG in the neighborhood of the nucleus. It is this product which is termed the nuclear quadrupole coupling constant for a given isotope in a material and can be found in tables of known NQR transitions.
Principle
Any nucleus with more than one unpaired nuclear particle (protons or neutrons) will have a charge distribution which results in an electric quadrupole moment. Allowed nuclear energy levels are shifted unequally due to the interaction of the nuclear charge with an electric field gradient supplied by the non-uniform distribution electron density (e.g. from bonding electrons) and/or surrounding ions. As in the case of NMR, irradiation of the nucleus with a burst of RF electromagnetic radiation, if of a particular frequency, results in absorption of some energy by the nucleus which can be viewed as a perturbation of the quadrupole energy level. Unlike the NMR case, NQR absorption takes place in the absence of an external magnetic field. Application of an external static field to a quadrupolar nucleus splits the quadrupole levels by the energy predicted from the Zeeman interaction . The technique is very sensitive to the nature and symmetry of the bonding around the nucleus. It can characterize phase transitions in solids when performed at varying temperature.Due to symmetry, the shifts become averaged to zero in the liquid phase, so NQR spectra can only be measured for solids.
Analogy with NMR
In the case of NMR, nuclei with spin ≥ 1/2 have a magnetic dipole moment so that their energies are split by a magnetic field, allowing resonance absorption of energy related to the Larmor frequency:
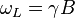
where 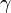 is the gyromagnetic ratio and
is the gyromagnetic ratio and 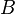 is the (normally applied) magnetic field external to the nucleus.
is the (normally applied) magnetic field external to the nucleus.
In the case of NQR, nuclei with spin ≥ 1, such as 14N, 17O, 35Cl and 63Cu, also have an electric quadrupole moment. The nuclear quadrupole moment is associated with non-spherical charge distributions, and is a measure of flatness or oblateness. NQR is a direct observation of the interaction of the quadrupole moment with the local electric field gradient (EFG) created by the electronic structure of its environment. The NQR transition frequencies are proportional to the product of the electric quadrupole moment of the nucleus and a measure of the strength of the local EFG:
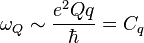
where q is a measure of our ignorance of the particular EFG due to the environment at the position of the nucleus.  is referred to as the quadrupole coupling constant.
is referred to as the quadrupole coupling constant.
In principle, the NQR experimenter could apply a specified EFG in order to influence 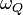 just as the NMR experimenter is free to choose the lamor frequency by adjusting the magnetic field. However, in solids, the strength of the EFG is many kV/m, making the application of EFG's for NQR in the manner that external magnetic fields are chosen for NMR impractical. Consequently, the NQR spectrum of a substance is specific to the substance - and NQR spectrum is a so called "chemical fingerprint." Because NQR frequencies are not chosen by the experimenter, they can be difficult to find making NQR a technically difficult technique to carry out. Since NQR is done in an environment without a static (or DC) magnetic field, it is sometimes called "zero field NMR". Many NQR transition frequencies depend strongly upon temperature.
just as the NMR experimenter is free to choose the lamor frequency by adjusting the magnetic field. However, in solids, the strength of the EFG is many kV/m, making the application of EFG's for NQR in the manner that external magnetic fields are chosen for NMR impractical. Consequently, the NQR spectrum of a substance is specific to the substance - and NQR spectrum is a so called "chemical fingerprint." Because NQR frequencies are not chosen by the experimenter, they can be difficult to find making NQR a technically difficult technique to carry out. Since NQR is done in an environment without a static (or DC) magnetic field, it is sometimes called "zero field NMR". Many NQR transition frequencies depend strongly upon temperature.
Applications
There are several research groups around the world currently working on ways to use NQR to detect explosives. Units designed to detect landmines[2] and explosives concealed in luggage have been tested. A detection system consists of a radio frequency (RF) power source, a coil to produce the magnetic excitation field and a detector circuit which monitors for a RF NQR response coming from the explosive component of the object.
A fake device known as the ADE 651 claimed to exploit NQR to detect explosives but in fact could do no such thing. Nonetheless, the device was successfully sold for millions to dozens of countries, including the government of Iraq.
Another practical use for NQR is measuring the water/gas/oil coming out of an oil well in realtime. This particular technique allows local or remote monitoring of the extraction process, calculation of the well's remaining capacity and the water/detergents ratio the input pump must send to efficiently extract oil.
Due to the strong temperature dependence of the NQR frequency, it can be used as a precise temperature sensor with resolution on the order of 10−4°C.[3]
References
- ↑
- ↑ Appendix K: Nuclear quadrupole resonance, by Allen N. Garroway, Naval Research Laboratory. In Jacqueline MacDonald, J. R. Lockwood: Alternatives for Landmine Detection. Report MR-1608, Rand Corporation, 2003.
- ↑ Leigh, James R. (1988). Temperature measurement & control. London: Peter Peregrinus Ltd. p. 48. ISBN 0-86341-111-8.