Neisseria gonorrhoeae
| Neisseria gonorrhoeae | |
|---|---|
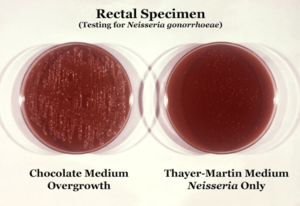 | |
| Neisseria gonorrhoeae cultured on two different media types. | |
| Scientific classification | |
| Kingdom: | Bacteria |
| Phylum: | Proteobacteria |
| Class: | Beta Proteobacteria |
| Order: | Neisseriales |
| Family: | Neisseriaceae |
| Genus: | Neisseria |
| Species: | N. gonorrhoeae |
| Binomial name | |
| Neisseria gonorrhoeae Zopf, 1885 | |
| Synonyms | |
|
Gonococcus Neisser 1879 | |
Neisseria gonorrhoeae, also known as gonococci (plural), or gonococcus (singular), is a species of Gram-negative coffee bean-shaped diplococci bacteria responsible for the sexually transmitted infection gonorrhea.[1]
N. gonorrhoeae was first described by Albert Neisser in 1879.
Microbiology
Neisseria are fastidious Gram-negative cocci that require nutrient supplementation to grow in laboratory cultures. To be specific, they grow on chocolate agar with carbon dioxide. These cocci are facultatively intracellular and typically appear in pairs (diplococci), in the shape of coffee beans. Of the eleven species of Neisseria that colonize humans, only two are pathogens. N. gonorrhoeae is the causative agent of gonorrhea (also called "The Clap") and is transmitted via sexual contact.[2]
Neisseria is usually isolated on Thayer-Martin agar (or VPN agar)—an agar plate containing antibiotics (vancomycin, colistin, nystatin, and trimethoprim) and nutrients that facilitate the growth of Neisseria species while inhibiting the growth of contaminating bacteria and fungi. Further testing to differentiate the species includes testing for oxidase (all clinically relevant Neisseria show a positive reaction) and the carbohydrates maltose, sucrose, and glucose test in which N. gonorrhoeae will oxidize (that is, utilize) only the glucose.
N. gonorrhoeae are non-motile and possess type IV pili to adhere to surfaces. The type IV pili operate mechanistically similar to a grappling hook. Pili extend and attach to a substrate that signals the pilus to retract, dragging the cell forward. N. gonorrhoeae are able to pull 100,000 times their own weight, and it has been claimed that the pili used to do so are the strongest biological motor known to date, exerting one nanonewton.[3]
N. gonorrhoeae has surface proteins called Opa proteins, which bind to receptors on immune cells. In so doing, N. gonorrhoeae is able to prevent an immune response. The host is also unable to develop an immunological memory against N. gonorrhoeae – which means that future reinfection is possible. N. gonorrhoeae can also evade the immune system through a process called antigenic variation, in which the N. gonorrhoeae bacterium is able to alter the antigenic determinants (sites where antibodies bind) such as the Opa proteins[4] and Type IV pili[5] that adorn its surface. The many permutations of surface proteins make it more difficult for immune cells to recognize N. gonorrhoeae and mount a defense.[6]

N. gonorrhoeae is naturally competent for DNA transformation as well as being capable of conjugation. These processes allow for the DNA of N. gonorrhoeae to acquire or spread new genes. Especially dangerous from the aspect of healthcare is the ability to conjugate, since this can lead to antibiotic resistance.[7]
Genome
The genomes of several strains of N. gonorrhoeae have been sequenced. Most of them are about 2.1 Mb in size and encode 2,100 to 2,600 proteins (although most seem to be in the lower range).[8] For instance, strain NCCP11945 consists of one circular chromosome (2,232,025 bp) encoding 2,662 predicted ORFs and one plasmid (4,153 bp) encoding 12 predicted ORFs. The estimated coding density over the entire genome is 87%, and the average G+C content is 52.4%, values that are similar to those of strain FA1090. The NCCP11945 genome encodes 54 tRNAs and four copies of 16S-23S-5S rRNA operons.[9]
In 2011, researchers at Northwestern University found evidence of a human DNA fragment in a Neisseria gonorrhoeae genome, the first example of horizontal gene transfer from humans to a bacterial pathogen.[10][11]
Transmission
N. gonorrhoeae is transmitted from person to person during sexual relations. Traditionally, the bacteria was thought to move attached to spermatozoon, but this hypothesis did not explain female to male transmission of the disease. A recent study suggests that rather than “surf” on wiggling sperm, N. gonorrhoeae bacteria uses hairlike structures called pili to anchor onto proteins in the sperm and move through coital liquid.[12] It can also be transmitted to the unborn infant in utero and afterward become apparent as a neonatal infection.
Disease
Symptoms of infection with N. gonorrhoeae differ, depending on the site of infection. Note also that 10% of infected males and 80% of infected females are asymptomatic.[13] Men who have had a gonorrhea infection have a significantly increased risk of having prostate cancer.[14]
Infection of the genitals can result in a purulent (or pus-like) discharge from the genitals, which may be foul-smelling. Symptoms may include inflammation, redness, swelling, and dysuria.
N. gonorrhoeae can also cause conjunctivitis, pharyngitis, proctitis or urethritis, prostatitis, and orchitis.
Conjunctivitis is common in neonates (newborns), and silver nitrate or antibiotics are often applied to their eyes as a preventive measure against gonorrhoea. Neonatal gonorrheal conjunctivitis is contracted when the infant is exposed to N. gonorrhoeae in the birth canal and can lead to corneal scarring or perforation, resulting in blindness in the neonate.
Disseminated N. gonorrhoeae infections can occur, resulting in endocarditis, meningitis or gonococcal dermatitis-arthritis syndrome. Dermatitis-arthritis syndrome presents with arthralgia, tenosynovitis, and painless non-pruritic (non-itchy) dermatitis.
Infection of the genitals in females with N. gonorrhoeae can result in pelvic inflammatory disease if left untreated, which can result in infertility. Pelvic inflammatory disease results if N. gonorrhoeae travels into the pelvic peritoneum (via the cervix, endometrium and fallopian tubes). Infertility is caused by inflammation and scarring of the fallopian tube. Infertility is a risk to 10 to 20% of the females infected with N. gonorrhoeae.
Treatment and prevention
If N. gonorrhoeae is resistant to the penicillin family of antibiotics, then ceftriaxone (a third-generation cephalosporin) is often used. Sexual partners should also be notified and treated.[15]
Antibiotic resistance in gonorrhea has been noted by beginning in the 1940s, when gonorrhea was treated with penicillin, but doses had to be continually increased in order to remain effective. By the 1970s, penicillin- and tetracycline-resistant gonorrhea emerged in the Pacific Basin. These resistant strains then spread to Hawaii, California, the rest of the United States, and Europe. Fluoroquinolones were the next line of defense, but soon resistance to this antibiotic emerged as well. Since 2007, standard treatment has been third-generation cephalosporins, such as ceftriaxone, which are considered to be our “last line of defense.”[16]
Recently, a high-level ceftriaxone-resistant strain of gonorrhea, called H041, was discovered in Japan. Lab tests found it to be resistant to high concentrations of ceftriaxone, as well as most of the other antibiotics tested. Within N. gonorrhoeae, there are genes that confer resistance to every single antibiotic used to cure gonorrhea, but thus far they do not coexist within a single gonococcus. Because of N. gonorrhoeae’s high affinity for horizontal gene transfer, however, antibiotic-resistant gonorrhea is seen as an emerging public health threat.[16]
Patients should also be tested for other sexually transmitted infections (there is a fivefold increase of HIV transmission[17]), especially Chlamydia infections, since co-infection is frequent (up to 50% of cases). Antibacterial coverage is often included for Chlamydia because of this.
Transmission can be reduced by the usage of latex barriers, such as condoms or dental dams, during intercourse, oral and anal sex, and by limiting sexual partners.
Vaccine
Due to the relative frequency of infection and the emerging development of antibiotic resistance in strains of N. gonorrhoeae, vaccines are thought to be an important goal in the prevention of infection. Development of a vaccine has been complicated by the emergence of resistant strains and strains that have the ability to alter the structure and composition of their cell membranes.[18]
Survival of gonococci
The exudates from infected individuals contain many polymorphonuclear leukocytes (PMN) with ingested gonococci. These gonococci stimulate the PMN to release an internal oxidative burst involving reactive oxygen species in order to kill the gonococci.[19] However, a significant fraction of the gonococci can resist killing and are able to reproduce within the PMN phagosomes.
Stohl and Seifert showed that the bacterial RecA protein, that mediates recombinational repair of DNA damage, plays an important role in gonococcal survival.[20] The protection afforded by RecA protein may be linked to transformation, the process by which recipient gonococci take up DNA from neighboring gonococci and integrate this DNA into the recipient genome through recombination. Michod et al. have suggested that an important benefit of transformation in N. gonorrhoeae may be recombinational repair of oxidative DNA damages caused by oxidative attack by the hosts phagocytic cells.[21]
See also
References
- ↑ Ryan, KJ; Ray, CG, eds. (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. ISBN 0-8385-8529-9.
- ↑ Genco, C; Wetzler, L, eds. (2010). Neisseria: Molecular Mechanisms of Pathogenesis. Caister Academic Press. ISBN 978-1-904455-51-6.
- ↑ Biais N, Ladoux B, Higashi D, So M, Sheetz M (2008). "Cooperative retraction of bundled type IV pili enables nanonewton force generation". PLoS Biol. 6 (4): e87. doi:10.1371/journal.pbio.0060087. PMC 2292754. PMID 18416602. Lay summary – New Scientist (19 April 2008).
- ↑ Stern, Anne; Brown, Melissa; Nickel, Peter; Meyer, Thomas F. (1986). "Opacity genes in Neisseria gonorrhoeae: Control of phase and antigenic variation". Cell 47 (1): 61–71. doi:10.1016/0092-8674(86)90366-1. PMID 3093085.
- ↑ Cahoon, Laty A.; Seifert, H. Steven (2011). "Focusing homologous recombination: Pilin antigenic variation in the pathogenic Neisseria". Molecular Microbiology 81 (5): 1136–43. doi:10.1111/j.1365-2958.2011.07773.x. PMID 21812841.
- ↑ STI Awareness: Gonorrhea. Planned Parenthood Advocates of Arizona. 11 April 2011. Retrieved 31 August 2011.
- ↑ Aas, Finn Erik; Wolfgang, Matthew; Frye, Stephan; Dunham, Steven; Løvold, Cecilia; Koomey, Michael (2002). "Competence for natural transformation in Neisseria gonorrhoeae: Components of DNA binding and uptake linked to type IV pilus expression". Molecular Microbiology 46 (3): 749–60. doi:10.1046/j.1365-2958.2002.03193.x. PMID 12410832.
- ↑ Neisseria gonorrhoeae genome statistics, Broad Institute
- ↑ Chung, G. T.; Yoo, J. S.; Oh, H. B.; Lee, Y. S.; Cha, S. H.; Kim, S. J.; Yoo, C. K. (2008). "Complete genome sequence of Neisseria gonorrhoeae NCCP11945". Journal of Bacteriology 190 (17): 6035–6. doi:10.1128/JB.00566-08. PMC 2519540. PMID 18586945.
- ↑ Anderson, Mark T.; Seifert, H. Steven (2014). "Neisseria gonorrhoeae and humans perform an evolutionary LINE dance". Mobile Genetic Elements 1 (1): 85–87. doi:10.4161/mge.1.1.15868. PMC 3190277. PMID 22016852. Lay summary – ScienceDaily (February 14, 2011).
- ↑ Anderson, M. T.; Seifert, H. S. (2011). "Opportunity and Means: Horizontal Gene Transfer from the Human Host to a Bacterial Pathogen". MBio 2 (1): e00005–11. doi:10.1128/mBio.00005-11. PMC 3042738. PMID 21325040.
- ↑ Anderson, M. T.; Dewenter, L.; Maier, B.; Seifert, H. S. (2014). "Seminal Plasma Initiates a Neisseria gonorrhoeae Transmission State". MBio 5 (2): e01004–13. doi:10.1128/mBio.01004-13. PMID 24595372.
- ↑ Detels, Roger; Green, Annette M.; Klausner, Jeffrey D.; Katzenstein, David; Gaydos, Charlotte; Hadsfield, Hunter; Peqyegnat, Willo; Mayer, Kenneth; Hartwell, Tyler D.; Quinn, Thomas . (2011). "The Incidence and Correlates of Symptomatic and Asymptomatic Chlamydia trachomatis and Neisseria gonorrhoeae Infections in Selected Populations in Five Countries". Sex. Transm. Dis. 38 (6): 503–509. PMID 3408314.
- ↑ Caini, Saverio; Gandini, Sara; Dudas, Maria; Bremer, Viviane; Severi, Ettore; Gherasim, Alin (2014). "Sexually transmitted infections and prostate cancer risk: A systematic review and meta-analysis". Cancer Epidemiology 38 (4): 329–338. doi:10.1016/j.canep.2014.06.002. ISSN 1877-7821. PMID 24986642.
- ↑ "UK doctors advised gonorrhoea has turned drug resistant". BBC News. 10 October 2011.
- 1 2 STI Awareness: Antibiotic-Resistant Gonorrhea. Planned Parenthood Advocates of Arizona. 6 March 2012. Retrieved 6 March 2012.
- ↑ Department of Reproductive Health and Research (2011). "Emergence of multi-drug resistant Neisseria gonorrhoeae – Threat of global rise in untreatable sexually transmitted infections" (PDF). FactSheet WHO/RHR/11.14. World Health Organization.
- ↑ Baarda, Benjamin I.; Sikora, Aleksandra E. (2015). "Proteomics of Neisseria gonorrhoeae: the treasure hunt for countermeasures against an old disease". Frontiers in Microbiology 6. doi:10.3389/fmicb.2015.01190. ISSN 1664-302X; Access provided by the University of Pittsburgh.
- ↑ Simons, M. P.; Nauseef, W. M.; Apicella, M. A. (2005). "Interactions of Neisseria gonorrhoeae with Adherent Polymorphonuclear Leukocytes". Infection and Immunity 73 (4): 1971–7. doi:10.1128/iai.73.4.1971-1977.2005. PMC 1087443. PMID 15784537.
- ↑ Stohl, E. A.; Seifert, H. S. (2006). "Neisseria gonorrhoeae DNA Recombination and Repair Enzymes Protect against Oxidative Damage Caused by Hydrogen Peroxide". Journal of Bacteriology 188 (21): 7645–51. doi:10.1128/JB.00801-06. PMC 1636252. PMID 16936020.
- ↑ Michod, Richard E.; Bernstein, Harris; Nedelcu, Aurora M. (2008). "Adaptive value of sex in microbial pathogens". Infection, Genetics and Evolution 8 (3): 267–85. doi:10.1016/j.meegid.2008.01.002. PMID 18295550.
External links
- Todar, Kenneth. "Pathogenic Neisseriae: Gonorrhea, Neonatal Ophthalmia and Meningococcal Meningitis". Todar's Online Textbook of Bacteriology.
- Gonorrhea at eMedicine
- "Neisseria gonorrhoeae". NCBI Taxonomy Browser. 485.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||