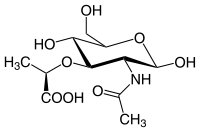
N-Acetylmuramic acid
 | |
| Identifiers | |
|---|---|
| 10597-89-4 | |
| ChEBI | CHEBI:21615 |
| ChemSpider | 4575341 |
| Jmol interactive 3D | Image |
| PubChem | 5462244 |
| |
| |
| Properties | |
| C11H19NO8 | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
N-Acetylmuramic acid, or MurNAc, is the ether of lactic acid and N-acetylglucosamine with a chemical formula of C11H19NO8. It is part of a biopolymer in the bacterial cell wall, built from alternating units of N-acetylglucosamine (GlcNAc) and N-acetylmuramic acid (MurNAc), cross-linked with oligopeptides at the lactic acid residue of MurNAc. This layered structure is called peptidoglycan.
MurNAc is a monosaccharide derivative of N-acetylglucosamine.
Clinical significance
Unlike most bacterial cell walls, Chlamydial cell wall lacks muramic acid. For this reason penicillin is not very effective in treating chlamydial infection. Protein synthesis blockers like doxycycline or azithromycin are used instead.
N-Acetylmuramic acid (MURNAc) is part of the peptidoglycan polymer of Gram-positive bacterial cell walls. MURNAc is covalently linked to N-acetylglucosamine and may also be linked through the hydroxyl on carbon number 4 to the carbon of L-alanine (Figure 9.26). A pentapeptide composed of L-alanyl-d--isoglutaminyl-L-lysyl-D-alanine-D-alanine is added to the MURNAc in the process of making the peptidoglycan strands of the cell wall.
Synthesis is inhibited by fosfomycin.[1]
References
- ↑ Grif K, Dierich MP, Pfaller K, Miglioli PA, Allerberger F (August 2001). "In vitro activity of fosfomycin in combination with various antistaphylococcal substances". Journal of antimicrobial chemotherapy 48 (2): 209–17. doi:10.1093/jac/48.2.209. PMID 11481290.
See also
| ||||||||||||||||||||||||||||||||||||