Baleen whale
| Baleen whales Temporal range: late Eocene–Recent | |
|---|---|
 | |
| Humpback whale breaching | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Artiodactyla |
| Clade: | Cetancodontamorpha |
| Suborder: | Whippomorpha |
| Infraorder: | Cetacea |
| Parvorder: | Mysticeti Cope 1891 |
| Families | |
|
see text | |
| Diversity | |
| 15 species | |
Mysticeti (/ˈmɪstʌsɛtiː/[1]) (from Greek ὁ μῦς τὸ κῆτος "the mouse, the whale so called" [2]), or baleen whales, are a widely distributed and diverse parvorder of carnivorous marine mammals. They comprise the families Balaenidae (right whales), Balaenopteridae (rorqual), Eschrichtiidae (the gray whale), and Cetotheriidae (the pygmy right whale). There are currently 15 species of baleen whale. While cetaceans were historically thought to have descended from mesonychids, molecular evidence supports them as relatives of even-toed ungulates (Artiodactyla). Baleen whale split from toothed whales (Odontoceti) around 34 million years ago.
Baleen whales range in size from the 20 ft (6 m) and 6,600 lb (3,000 kg) pygmy right whale to the 112 ft (34 m) and 190 t (210 short tons) blue whale, which is also the largest creature. They exhibit sexual dimorphism. Baleen whales can have streamlined or large bodies, depending on the feeding behavior, and two limbs that are modified into flippers. Though not as flexible and agile as seals, baleen whales can swim very fast. Baleen whales use their plates to filter out food from the water by either lunge-feeding or gulp-feeding. Baleen whales have fused neck vertebrae, and are unable to turn their head at all. Baleen whales have two blowholes. Some species are well adapted for diving to great depths. They have a layer of fat, or blubber, under the skin to keep warm in the cold water.
Although baleen whales are widespread, most species prefer the colder waters around the Northern and Southern Poles. They spend their lives in the water, mating, giving birth, and molting completely submerged, only coming to the surface to breathe. Gray whales are specialized for feeding on bottom-dwelling mollusks. Rorquals are specialized at lunge-feeding, and have a streamlined body to reduce drag while accelerating. Right whales gulp-feed, meaning they use their enlarged head to effectively take in a large amount of water and sieve the slow-moving prey. Males typically mate with more than one female (polygyny), although the degree of polygyny varies with the species. Male strategies for reproductive success vary between performing ritual displays (whale song) or lek mating. Calves are typically born in the spring and summer months and females bear all the responsibility for raising them. Mothers fast and nurse their young for a relatively long period of time. Baleen whales produce a number of vocalizations, notably the songs of the humpback whale.
The meat, blubber, baleen, and oil of baleen whales have traditionally been used by the indigenous peoples of the Arctic. Once relentlessly hunted by commercial industries for these products, cetaceans are now protected by international law. However, the North Atlantic right whale is ranked critically endangered by the International Union for Conservation of Nature. Besides hunting, baleen whales also face threats from marine pollution, ocean acidification, and naval sonar, which results in strandings.
Etymology
The taxonomic name "Mysticeti" (Greek, plural) apparently derives from a translation error in early copies of Aristotle's Historia Animalium, in which "ὁ μῦς τὸ κῆτος" (ho mus to kētos, "the mouse, the whale so called") was mistakenly run together as "ὁ μυστικῆτος" (ο mustikētos, "the Mysticetus"),[2] which Rice 1998 assumed was an ironic reference to the animals' great size.[3] An alternate name for the suborder is "Mystacoceti" (from Greek μύσταξ "moustache" + κῆτος "whale"), which, although obviously more appropriate and occasionally used in the past, has been superseded by "Mysticeti".[3]
The term "baleen" (Middle English baleyn, ballayne, ballien, bellane, etc.) is an archaic word for "whale", derived from the Latin balæna.[4]
Taxonomy
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cladogram showing phylogenic relations between mysticete species according to Hassanin and Ropiquet, et al.,[5] Sasaki and Nikaido, et al.,[6] and Rosenbaum and Brownell, Jr., et al.[7] |
Mysticetes are also known as baleen whales due to the presence of baleen. These animals rely on their baleen plates to sieve plankton and other small organisms from the water. Mysticetes consist of four families: Balaenidae (right whales), Balaenopteridae (rorquals), Cetotheriidae (pygmy right whale), and Eschrichtiidae (gray whale). Balaenids are distinguished by their enlarged head and thick blubber,[8][9] while rorquals and gray whales generally have a flat head, long throat pleats,[10] and are more streamlined than Balaenids. Rorquals also tend to be larger than the latter. Two genera and nine species of rorqual are known to exist, while two genera and three species of Balaenid exist.
Eschrichtiidae consists of only one living member: the gray whale. This animal is easily distinguished from other extant cetaceans by its sleet-gray color, a dorsal ridge (knuckles on the back), and its gray-white scars left from parasites. Like in the rorquals, their throat pleats increase the capacity of their throat, allowing them to filter larger volumes of water at once. Gray whales are bottom-feeders, meaning they sift through sand to get their food. They usually turn on their side and scoop up sediment into their mouth and filter out benthic creatures like amphipods.[11] In addition, the two populations, one in the Sea of Okhotsk and Sea of Japan and the other in the Mediterranean Sea[12] and East Atlantic,[13] are thought to be genetically and physiologically dissimilar.[14] The gray whale is traditionally placed as the only living species in its genus and family. However, DNA analysis by studies, such as Sasaki and Nikaido, et al.,[6] indicates certain rorquals, such as the humpback whale, Megaptera novaeangliae, and the fin whale, Balaenoptera physalus, are more closely related to the gray whale than they are to some other rorquals, such as the minke whale.[15]
Rorquals consist of two genera (Balaenoptera and Megaptera) and nine species: the fin whale, the Sei whale, Bryde's whale, Eden's whale, the blue whale, the common minke whale, the Antarctic minke whale, Omura's whale, and the humpback whale. Rorquals are different from other mysticetes in that they use throat pleats to expand their mouth which allows them to feed more effectively. However, rorquals need to build up water pressure in order to expand their mouth, leading to a lung-feeding behavior. Lung-feeding is where a whale rams a bait ball at high speeds and opens its mouth as it hits the bait ball, to reduce drag while accelerating. Rorquals generally have a streamlined physique to reduce drag in the water while foraging.[16] In a 2012 review of cetacean taxonomy, Alexandre Hassanin and Anne Ropiquet, et al., suggested that, based on phylogenic criteria, there are 4 extant genera of rorquals. They recommend that the genus Balaenoptera be limited to the fin whale, and have minke whales fall under the genus Pterobalaena, and have Rorqualus contain the Sei whale, Bryde's whale, Eden's whale, the blue whale, and Omura's whale.[5]
Balaenids are also known as right whales due to whalers preferring them over other species; they were essentially the "right whale" to catch.[17] These animals rely on their huge head, as opposed to the rorquals' throat pleats, to feed effectively. This feeding behavior allows them to grow very big and bulky, without the necessity for a streamlined body. They usually target slow baitballs of prey that can be filtered through their baleen plates. Balaenids consist of two genera: Eubalaena (right whales) and Balaena (the bowhead whale). Balaenidae originally consisted of only one genus until recent studies done through the early 2000s reported that bowhead whales and right whales are morphologically and phylogenically different. According to a study done by Rosenbaum and Brownell, Jr., et al., the North Pacific and Southern right whales are more closely related to each other than to the North Atlantic right whale.[7]
Cetotheriidae consists of only one living member: the pygmy right whale. This animal is easily confused with minke whales due to their similar characteristics. The pygmy right whale is very elusive, rarely seen in the wild and, consequently, little is known about its feeding behavior and much of what is known about them comes from stranded individuals. Despite its name, the pygmy right whale is more genetically similar to rorquals and gray whales than to other right whales, named simply because its bones resemble that of right whales.[18] A study published in 2012 that, based on bone structure, moved the pygmy right whale from the (now empty) family Neobalaenidae to the family Cetotheriidae, making it a living fossil.[19]
- The "†" signs denote extinct families and genera.
- Parvorder Mysticeti: baleen whales
- Family †Aetiocetidae[20]
- †Aetiocetus
- †Ashorocetus
- †Chonecetus
- †Fucaia[21]
- †Morawanocetus
- †Willungacetus
- †Family Llanocetidae
- †Family Mammalodontidae
- Clade Chaeomysticeti
- Superfamily Eomysticetoidea
- †Family Cetotheriopsidae
- †Family Eomysticetidae
- †Eomysticetus
- †Micromysticetus
- †Tohoraata
- †Tokarahia
- †Waharoa
- †Yamatocetus
- Clade Balaenomorpha
- Superfamily Balaenoidea
- Family Balaenidae: right whales and bowhead whale
- Balaena – bowhead whales
- †Balaenella
- †Balaenotus
- †Balaenula
- Eubalaena – right whales
- †Idiocetus
- †Morenocetus
- †Peripolocetus
- Family Balaenidae: right whales and bowhead whale
- Clade Thalassotherii
- †Hibacetus
- †Isocetus
- Family Cetotheriidae
- †Brandtocetus
- †Cephalotropis
- †Cetotherium
- †Eucetotherium
- †Herentalia
- †Herpetocetus
- †Joumocetus
- †Kurdalagonus
- †Metopocetus
- †Nannocetus
- †Otradnocetus
- †Palaeobalaena?
- †Piscobalaena
- †Titanocetus?
- †Vampalus
- †Zygiocetus
- Subfamily Neobalaeninae[19]
- Superfamily Balaenopteroidea
- †Eobalaenoptera
- †Mauicetus
- †Tiphyocetus
- Family †Aglaocetidae
- † Aglaocetus
- † Isanacetus
- † Pinocetus
- Family Balaenopteridae: rorquals[22]
 Rorqual skeleton with unfused mandibular symphysis clearly visible
Rorqual skeleton with unfused mandibular symphysis clearly visible- †Archaebalaenoptera
- Balaenoptera
- †Burtinopsis (nomen dubium)[23]
- †Cetotheriophanes
- †Diunatans
- Megaptera – humpback whale
- †Notiocetus
- †Parabalaenoptera
- †Plesiobalaenoptera
- †Plesiocetus
- †Praemegaptera
- †Protororqualus
- †Family Diorocetidae
- †Amphicetus
- †Diorocetus
- †Plesiocetopsis
- †Thinocetus
- †Uranocetus
- Subfamily Eschrichtiinae[24]
- †Archaeschrichtius
- †Eschrichtioides
- Eschrichtius – gray whales
.jpg) Gray whale skeleton
Gray whale skeleton - †Gricetoides
- †Megapteropsis (nomen dubium)[20]
- †Family Pelocetidae (invalid subgroup)[20]
- †Cophocetus
- †Halicetus
- †Parietobalaena
- †Pelocetus
- †Family Tranatocetidae
- Superfamily Balaenoidea
- Superfamily Eomysticetoidea
- Family incertae sedis
- Family †Aetiocetidae[20]
- Parvorder Mysticeti: baleen whales
Evolutionary history

Mysticeti split from Odontoceti (toothed whales) 34 mya during the Eocene.[26] Their evolutionary link to archaic toothed cetaceans (Archaeoceti) remained unknown until Janjucetus hunderi was discovered in the early 1990s in Victoria. Like a modern baleen whale, Janjucetus had baleen present in its jaw and had very little biosonar capabilities. However, its jaw also contained teeth, with incisors and canines built for stabbing and molars and premolars built for tearing. These early mysticetes were exceedingly small compared to modern baleen whales, with species like Mammalodon measuring no greater than 10 feet (3.0 m). It is thought that size and baleen dependence are linked.[27] The discovery of Janjucetus and others like it suggests that baleen evolution went through several transitional phases.[28]

Originally thought to be Llanocetus, Fucaia buelli is the earliest mysticete, dating back to 33 mya. Measuring only 6.6 feet (2 m), it is the smallest extant baleen whale. It is only known from its teeth; they suggest a suction-feeding behavior, much like that of beaked whales. Like other early toothed mysticetes, F. buelli had heterodont dentition.[21] Other early toothed mysticeti or "archaeomysticetes" from the Oligocene are the Mammalodontidae (Mammalodon and Janjucetus) from Australia. They are small with shortened rostra, and a primitive dental formula (3.1.4.33.1.4.3).[29] In baleen whales, enlarged oral cavities adapted for suction feeding evolved before specializations for bulk filter feeding. In the toothed Oligocene mammalodontid Janjucetus, the symphysis is short and the oral cavity enlarged, the rostrum is wide, and the edges of the maxillae are thin, indicating an adaptation for suction feeding. The aetiocetid Chonecetus still had teeth, but the presence of a groove on the interior side of each mandible indicates the symphysis was elastic, which would have enabled rotation of each mandible, an initial adaptation for bulk feeding like in modern mysticetes.[30]
The lineages of rorquals and right whales split almost 20 mya. Is is unknown where this occurred, but it is largely thought that they, like their descendants, followed plankton migrations. These primitive mysticetes had lost their heterodont dentition in favor of baleen, and are thought to have lived on a specialized benthic, plankton, or copepod diet like modern mysticetes. Mysticetes experienced their first radiation in the mid-Miocene. Balaenopterids got bigger during this time, with species like Balaenoptera sibbaldina rivaling the blue whale in terms of size.[31] It is thought this radiation was caused by global climate change and major tectonic activity (the Antarctic Circumpolar Current).[32]

The first toothless ancestors of Mysticetes appeared before the first radiation in the late Oligocene.[33] Eomysticetus and others like it showed no evidence in the skull of echolocation abilities, suggesting they mainly relied on their eyesight for navigation. The Eomysticetidae had long, flat rostra that lacked teeth and had external nares located halfway up the dorsal side of the snout. Though the palate is not well-preserved in these specimens, they are thought to have had baleen and been filter feeders.[29][34]
Anatomy
Baleen whales have two flippers on the front, near the head. The hind legs are enclosed inside the body, and are thought to be vestigial organs. However, a study done in 2014 suggests that the pelvic bone serves as support for whale genitalia.[35] Due to their great size, baleen whales are not flexible or agile like dolphins, and none can move their neck due to the fused cervical vertebrae; this sacrifices speed for stability in the water.[36]
Rorquals, needing to build speed to feed, have several adaptions for reducing drag, including a streamlined body, a small dorsal fin relative to its size, and lack of external ears or hair. While feeding, the rorqual jaw expands to a volume that can be bigger than the whale itself; to do this, the oral cavity inflates to expand the mouth. The inflation of the oral cavity causes the cavum ventrale, the folds (throat pleats) on the throat stretching to the naval, to expand, increasing the amount of water that the mouth can store.[37] The mandible is connected to the skull by dense fibers and cartilage, allowing the jaw to swing open at almost a 90° angle. The mandibular symphysis is also fibrocartilaginous, allowing the jaw to bend which lets in more water.[38] To prevent stretching the mouth too far, rorquals have a sensory organ located in the middle of the jaw to regulate these functions.[39]
When swimming, baleen whales rely on their flippers for locomotion in a wing-like manner similar to penguins and sea turtles. Flipper movement is continuous. While doing this, baleen whales use their tail fluke to propel themselves forward through vertical motion while using their flipper for steering, much like an otter. Some species leap out of the water, which may allow then to travel faster.[40]


Like all mammals, baleen whales breathe air and must surface periodically to do so. Their nostrils, or blowholes, are situated at the top of the cranium. The baleen whales have two blowholes, as opposed to toothed whales which have one. These paired blowholes are longitudinal slits that converge anteriorly and widen posteriorly, which causes a V-shaped blow. They are surrounded by a fleshy ridge that keeps water away while the whale breathes. The septum that separates the blowholes has two plugs attached to it, making the blowholes water-tight while the whale dives.[41]
When sieved from the water, food is swallowed and travels through the esophagus where it meets a three-chambered-stomach. The first compartment is known as the fore-stomach; this is where food gets ground up into an acidic liquid, which is then squirted into the main stomach. Like in humans, the food is mixed with hydrochloric acid and protein-digesting enzymes. Then, the partly digested food is moved into the third stomach, where it meets fat-digesting enzymes, and is then mixed with an alkaline liquid to neutralize the acid from the first stomach to prevent damage to the intestinal tract. The whale intestinal track is highly adapted to absorb the most nutrients from food; the walls are folded and contain copious blood vessels, allowing for a greater surface area over which digested food and water can be absorbed. Whales get the water they need from their food, however the salt content of most of their prey (invertebrates) are similar to that of seawater, whereas the salt content of a whale's blood is considerably lower (three times lower) than that of seawater. The whale kidney is adapted to excreting excess salt, however, while producing urine more concentrated than seawater, it wastes a lot of water which must be replaced.[42]
Senses
The eyes of baleen whales are relatively small for their size and are positioned near the end of the mouth. This is probably because they feed on slow or immobile prey, and that most sunlight does not pass 30 feet (9.1 m), and hence do not need acute vision. A whale's eye is adapted for seeing both in the euphotic and aphotic zones by increasing or decreasing the pupil's size to prevent damage to the eye. As opposed to land mammals, whales have spherical eyeballs. The retina is surrounding by reflective layer of cells (tapetum lucidum), which bounces back at the retina, enhancing eyesight in dark areas. However, light is bent more near the surface of the eye when in air as opposed to water; consequently, they can see much better in the air than in the water. The eyeballs are protected by a thick outer layer to prevent abrasions, and an oily fluid (instead if tears) on the surface of the eye. Baleen whales appear to have limited color vision, as they lack S-cones.[43]

The mysticete ear is adapted for hearing underwater, where it can hear sound frequencies as low as 7 Hz and as high as 22 kHz,[44] with the exception of the 52-hertz whale.[45] It is largely unknown how sound is received by baleen whales. Unlike in toothed whales, sound does not pass through the lower jaw. The auditory meatus is blocked by connective tissue and an ear plug, which connects to the eardrum. The inner-ear bones are contained in the tympanic bulla, a bony capsule. However, this is attached to the skull, suggesting that vibrations passing through the bone is important. Sinuses may reflect vibrations towards the cochlea. It is known that when the fluid inside the cochlea is disturbed by vibrations, it triggers sensory hairs which send electrical current to the brain, where vibrations are processed into sound.[46][47]
It is largely unknown how baleen whales produce sound due to the lack of a melon and vocal cords. In a 2007 study, it was discovered that the larynx had U-shaped folds which are thought to be similar to vocal cords. They are positioned parallel to air flow, as opposed to the perpendicular vocal cords of terrestrial mammals. These may control air flow and cause vibrations. The walls of the larynx are able to contract which may generate sound with support from the arytenoid cartilages. The muscles surrounding the larynx may expel air rapidly or maintain a constant volume while diving.[48]
Baleen whales have a small, yet functional, vomeronasal organ. This allows baleen whales to detect chemicals and pheromones released by their prey. It is thought that tasting the water is important for finding prey, and track down other whales. They are thought to have an impaired sense of smell due to the lack of the olfactory bulb, but they do have an olfactory tract.[49] Baleen whales have little to no taste buds, suggesting they have lost their sense of taste.[50]
Diving adaptations
Before diving, baleen whales typically stay near the surface and take 10 to 15 breaths for around three minutes and dive for eight minutes. Their unique lungs are built to collapse under the pressure instead of resisting the pressure which would damage the lungs. The whale lungs are very efficient at extracting oxygen from the air, usually 80%, whereas humans only extract 20% of oxygen from inhaled air. Lung volume is relatively low compared to terrestrial mammals due to the inability of the respiratory tract to hold gas while diving. Doing so may cause serious complications such as embolism. Unlike other mammals, the lungs of baleen whales lack lobes and are more sacculated shaped. Like in humans, the left lung is smaller than the right to make room for the heart.[51] To conserve oxygen, blood is rerouted from pressure-tolerant-tissue to internal organs,[52] and they have a high concentration of myoglobin which allows them to hold their breath longer.[53]
Thermoregulation
Baleen whales conserve heat with their large and compact body size, and insulating blubber. In addition, they have a dense network of blood vessels (rete mirabile) which prevents heat-loss. In most mammals, heat is lost in their extremities, so warm blood in arteries is surrounded by veins to prevent heat loss during transport. As well as this, heat inevitably given off by the arteries warms blood in the surrounding veins as it travels back into the core. This is otherwise known as countercurrent exchange. To counteract overheating while in warmer waters, whales reroute blood to the skin to accelerate heat-loss.[54]
Sleep
Unlike most animals, whales are conscious breathers. All mammals sleep, but whales cannot afford to become unconscious for long because they may drown. They are thought to exhibit unihemispheric slow-wave sleep, in which they sleep with half of the brain while the half remains active. This behavior was only documented in toothed whales until footage of a humpback whale sleeping (vertically) was shot in 2014.[55]
Behavior
Migration
It is thought that plankton blooms dictate where whales migrate. This usually occurs in the polar regions during the sunny spring and summer months, bringing along other plankton such as Euphausiids which whales feed on. They also migrate to calving grounds in tropical waters during the winter months when plankton populations are low. As well as this, newborns, with underdeveloped blubber, would likely die of frostbite in the winter temperatures.[56] It is also postulated that these take place to avoid calves being predated on by killer whales.[57] The migration cycle is repeated annually.[58] The gray whale has the longest recorded migration of any mammal, with one travelling 14,000 miles (23,000 km) from the Sea of Okhotsk to the Baja peninsula.[59]
Foraging

All baleen whales are carnivorous, however a study done in 2015 revealed they house gut flora similar to that of terrestrial herbivores.[60] Different kinds of prey are found in different abundances depending on location, for example, Antarctic residents mostly feed on Euphausiids, however this is mainly effective for lunge-feeders, whereas gulp-feeders, like the right whales, feed primarily on copepods. Each type of whale is adapted to a specialized way of foraging. They feed solitarily or in small groups. There are two types of feeding behaviors: gulp-feeding and lunge-feeding, but some species do both depending on the type and amount of food.[61] Baleen whales get the water they need from their food, and their kidneys excrete excess salt.[42]
Lunge-feeders are all classified under the families Balaeonopteridae (rorquals) and Cetotheriidae (pygmy right whale). To feed, lunge-feeders expand the volume of their jaw to a volume bigger than the original volume of the whale itself; to do this, the oral cavity inflates to expand the mouth. The inflation of the oral cavity causes the throat pleats to expand, increasing the amount of water that the mouth can store.[37] Just before they ram the baitball, the jaw swings open at almost a 90° angle and bends which lets in more water.[38] To prevent stretching the mouth too far, rorquals have a sensory organ located in the middle of the jaw to regulate these functions.[39] They then must decelerate. This process takes a lot of mechanical work, and is only energy-effective when used against a large baitball.[62] Gulp-feeders, or skim-feeders, are all classified under the family Balaenidae (right whales) and Eschrichtiidae (gray whale). To feed, gulp-feeders swim with an open mouth, filling it with water and prey. Prey must occur in sufficient numbers to trigger the whale's interest, be within a certain size range so that the baleen plates can filter it, and be slow enough so that it cannot escape. The "skimming" may take place on the surface, underwater, or even at the ocean's bottom, indicated by mud occasionally observed on right whales' bodies. Gray whales feed primarily on the ocean's bottom, feeding on benthic creatures.[63]
Predation and parasitism

Baleen whales, due to their great size, do not have any natural predators. However, calves can be preyed on by the killer whale. It is thought that annual whale migration occurs to protect the calves from the killer whales.[57] There have also been reports of a pod attacking and killing an adult bowhead whale, holding down its flippers, covering the blowhole, ramming, and biting until dead.[64] Generally, a mother and calf pair, when faced with the threat of a killer whale pod, will either fight or flee. The fleeing response will only occur in species that can swim away quickly: the rorquals. For the slower whales, they must fight the pod alone or with a small family group.[65] Large sharks are another predator of whale calves. Sharks rarely attack whale calves, and there has been only one report of a shark attacking and killing a whale calf. This occurred in 2014 during the sardine run when a shiver of dusky sharks attacked a humpback whale calf.[66] Usually, the only shark that will go after a whale is the cookie cutter shark, which leaves a small, nonfatal bite mark.[67]
Many parasites latch onto whales, notably whale lice and whale barnacles. Though not a parasite, whale barnacles latch onto the skin of a whale during its larval stage. However, in doing so it does not harm nor benefit the whale. Due to this, their relationship is often labeled as an example of commensalism.[68] Almost all species of whale lice are specialized towards a certain species of whale, and there can be more than one species per whale. Whale lice eat dead skin, resulting in minor wounds in the skin. Whale louse infestations are especially evident in right whales, where colonies propagate on their callosities.[69] A species of copepod, Balaenophilus unisetus, inhabits baleen plates of whales in tropical waters. A species of Antarctic diatom, Cocconeis ceticola, forms a film on the skin, which takes a month to develop. They are also plagued by internal parasites such as stomach worms, cestodes, nematodes, liver flukes, and acanthocephalans.[70]
Reproduction and development

Before reaching adulthood, baleen whales grow at an extraordinary rate. In the blue whale, the largest species, the fetus grows by some 220 lb (100 kg)/day just before delivery, and by 180 lb (80 kg)/day during suckling. Before weaning, the calf increases its body weight by 17 t (17 long tons; 19 short tons) and grows from 23 to 26 ft (7 to 8 m) at birth to 43 to 52 ft (13 to 16 m) long. When it reaches sexual maturity after 5–10 years, it will be 66 to 79 ft (20 to 24 m) long and possibly live as long as 80–90 years.[71] Calves are born precocial, needing to be able to swim to the surface at the moment of its birth.
The same life pattern can be seen in other balaenopterids; they mate in warm waters in winter to give birth almost a year later.[58] A 7-to-11 month lactation period is normally followed by a year of rest before mating starts again. Adults normally start reproducing when 5–10 years old and reach their full length after 20–30 years.[72][73][74] In the smallest balaenopterid, the minke whale, 10 ft (3.0 m) calves are born after a 10-month pregnancy and weaning lasts until it has reached about 16 to 18 ft (5 to 5.5 m) after 6–7 months.[75] Unusual for a baleen whale, female minkes (and humpbacks) can become pregnant immediately after giving birth; in most species, there is a 2-to-3-year calving period. In right whales, the calving interval is usually 3 years. They grow very rapidly during their first year, after which they hardly increase in size for several years.[76] They reach sexual maturity when 43 to 46 ft (13 to 14 m) long. Baleen whales are K-strategists, meaning they raise one calf at a time, have a long life-expectancy, and a low infant mortality rate.[77] Some 19th-century harpoons found in harvested bowheads indicate this species can live more than 100 years.[78] Baleen whales are promiscuous, with none showing pair bonds.[75]
Whale song

 |
Singing Humpbacks
Recording of Humpback Whales singing and clicking |
| Problems playing this file? See media help. | |
All baleen whales use sound for communication and are known to "sing", especially during the breeding season. Blue whales produce the loudest sustained sounds of any animals: their low-frequency (about 20 Hz) moans can last for half a minute, reach almost 190 decibels, and be heard hundreds of kilometers away. Adult male humpbacks produce the longest and most complex songs; sequences of moans, groans, roars, sighs, and chirps sometimes lasting more than ten minutes are repeated for hours. Typically, all humpback males in a population sing the same song over a breeding season, but the songs change slightly between seasons, and males in one population have been observed adapting the song from males of a neighbouring population over a few breeding seasons.[79]
Intelligence
Unlike their toothed whale counterparts, baleen whales are hard to study due to their immense size. Intelligence tests such as the mirror test cannot be done because their bulk and lack of body language makes a reaction impossible to be definitive. However, studies on the brains of humpback whales revealed spindle cells, which, in humans, control theory of mind. Due to this, it is thought that baleen whales, or at least humpback whales, have consciousness.[80]
Relationship with humans
History of whaling
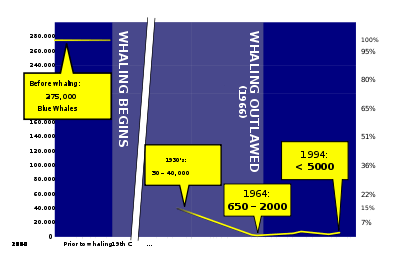
Whaling by humans has existed since the Stone Age. Ancient whalers used harpoons to spear the bigger animals from boats out at sea.[81] People from Norway started hunting whales around 4,000 years ago, and people from Japan began hunting whales in the Pacific at least as early as that.[82] Whales are typically hunted for their meat and blubber by aboriginal groups; they used baleen for baskets or roofing, and made tools and masks out of bones.[82] The Inuit hunted whales in the Arctic Ocean.[82] The Basques started whaling as early as the 11th century, sailing as far as Newfoundland in the 16th century in search of right whales.[83][84] 18th and 19th century whalers hunted down whales mainly for their oil, which was used as lamp fuel and a lubricant and baleen or whalebone, which was used for items such as corsets and skirt hoops,[82] The most successful whaling nations at this time were the Netherlands, Japan, and the United States.[85]
Commercial whaling was historically important as an industry well throughout the 17th, 18th and 19th centuries. Whaling was at that time a sizeable European industry with ships from Britain, France, Spain, Denmark, the Netherlands and Germany, sometimes collaborating to hunt whales in the Arctic.[86] By the early 1790s, whalers, namely the Americans and Australians, mainly focused efforts in the South Pacific where they mainly hunted right whales, with catches of up to 39,000 right whales by Americans alone.[87] At its height in the 1880s, U.S. profits turned to USD10,000,000, equivalent to USD225,000,000 today. Commonly exploited species included arctic whales, such as the gray whale, right whale, and bowhead whale, because they were close to the main whaling ports (like New Bedford). After those stocks were depleted, rorquals in the South Pacific were targeted by nearly all whaling organizations, however they often out-swam whaling vessels. Whaling rorquals was not effective until the harpoon cannon was invented in the late 1860s.[88] Whaling basically stopped when stocks of all species were depleted to a point that they could not be harvested on a commercial scale.[89] Whaling was controlled in 1982 when the International Whaling Commission (IWC) placed a moratorium setting catch limits to protect species from dying out from over-exploitation, and eventually banned it.
Notwithstanding the other provisions of paragraph 10, catch limits for the killing for commercial purposes of whales from all stocks for the 1986 coastal and the 1985/86 pelagic seasons and thereafter shall be zero. This provision will be kept under review, based upon the best scientific advice, and by 1990 at the latest the Commission will undertake a comprehensive assessment of the effects of this decision on whale stocks and consider modification of this provision and the establishment of other catch limits.
–IWC Commission Schedule, paragraph 10(e)[90]
Conservation and management issues
As of 2013, the International Union for Conservation of Nature (IUCN) recognizes 15 mysticete species. One species—the North Atlantic right whale—is endangered with only around 400(±50) individuals left, and four more are also classified as Endangered" (North Pacific right whale, the blue whale, the fin whale, and the Sei whale), and another 5 ranked as Data deficient (Bryde's whale, Eden's whale, Omura's whale, Southern minke whale, and pygmy right whale).[91] Species that live in polar habitats are vulnerable to the effects of recent and ongoing climate change, particularly declines in sea ice, as well as ocean acidification.[92]
The whale watching industry and anti-whaling advocates argue that whaling catches "friendly" whales that are curious about boats, as these whales are the easiest to catch. This analysis claims that once the economic benefits of hotels, restaurants and other tourist amenities are considered, hunting whales is a net economic loss. This argument is particularly contentious in Iceland, as it has among the most-developed whale-watching operations in the world and the hunting of minke whales resumed in August 2003. Brazil, Argentina and South Africa argue that whale watching is a growing billion-dollar industry that provides more revenue than commercial whaling would provide.[93] Peru, Uruguay, Australia, and New Zealand also support proposals to permanently forbid whaling South of the Equator, as Solor (an island of Indonesia) is the only place of the Southern Hemisphere that takes whales.[94] Anti-whaling groups claim that developing countries which support a pro-whaling stance are damaging their economies by driving away anti-whaling tourists.[95]

Commercial whaling was historically important for world economy. All species were exploited, and as one type's stock depleted, another type was targeted. The scale of whale harvesting decreased substantially through the 1960s as all whale stocks had been depleted, and practically stopped in the 1988 after the International Whaling Commission placed a moratorium which banned whaling for commercial use.[96] Several species that were commercially exploited have rebounded in numbers; for example, gray whales may be as numerous as they were prior to whaling, making it the first marine mammal to be taken off the Endangered species list.[97] The Southern right whale was hunted to near extinction in the mid-to-late 20th century, with only a small (unknown) population around Antarctica. Due to international protection, the Southern right whale's population has been growing 7% annually since 1970.[98] Conversely, the eastern stock of North Atlantic right whale was extirpated from much of its former range, which stretched from the coast of North Africa to the North Sea and Iceland; it is thought that the entire stock consists of only ten individuals, making the eastern stock functionally extinct.[96][99]
Baleen whales continue to be harvested. However, only three nations take whales: Iceland, Norway, and Japan. All these nations are part of the IWC, with Norway and Iceland rejecting the moratorium and continue commercial whaling.[100] Japan, being part of the IWC, whales under the Scientific Permit stated in Article VIII in the Convention for the Regulation of Whaling.[101] Japan has had two main research programs: JARPA and JARPN. JARPN is focused in the North Pacific and JARPA around the Antarctic. JARPA mainly caught Antarctic minke whales, catching nearly 7,000; to a far lesser extent, they also caught fin whales.[102] Animal-rights activist groups (such as the Greenpeace[103]) object to Japan's scientific whaling, with some calling it a substitute for commercial whaling.[104] In 2014, the United Nations judicial branch banned the taking of whales for any purpose in the Southern Ocean Whale Sanctuary,[105] however Japan refuses to stop whaling and has only promised to cut their annual catches by a third (around 300 whales per year).[106]

Baleen whales can also be effected by humans in more indirect ways. For species like the North Atlantic right whale, which migrates through some of the world's busiest shipping lanes, the biggest threat is from being struck by ships. The Lloyd's mirror effect results in low frequency propeller sounds not being discernible near the surface, where most accidents occur. Combined with spreading and acoustic shadowing effects, the result is that the whale is unable to hear an approaching vessel before it has been run over or entrapped by the hydrodynamic forces of the vessel's passage.[107] The ever-increasing amount of ocean noise, including sonar, drowns out the vocalizations produced by whales, notably in the blue whale which produces the loudest vocalization, which makes it harder for them to communicate.[108][109] Blue whales stop producing foraging D calls once a mid-frequency sonar is activated, even though the sonar frequency range (1–8 kHz) far exceeds their sound production range (25–100 Hz).[110] Poisoning from toxic substances such as Polychlorinated biphenyl (PCB) is generally low due to their low trophic level.[111] Some baleen whales can become victims of bycatch, which is especially serious for North Atlantic right whales, considering there are only 450 left.[112] Right whales feed with wide-open mouths, risking entanglement in any rope or net fixed in the water column. Rope wraps around their upper jaws, flippers and tails. Some are able to escape, but others remain entangled. If observers notice, they can be successfully disentangled, but others die over a period of months. Other whales, such as humpback whales, can also be entangled.[113]
In captivity

Baleen whales have rarely been kept in captivity. Their large size and appetite make them expensive creatures to maintain. Pools of proper size would also be very expensive to build. For example, a single gray whale calf would need to eat 475 pounds (215 kg) of fish per day, and the pool would have to accommodate the 13-foot (4.0 m) calf, along with ample room to swim.[114] Only two species have survived being kept in captivity for over a year before being released: gray whales and minke whales. The first gray whale, who was captured in Scammon's Lagoon, Baja California, in 1965, was named Gigi and died two months later from an infection.[115] The second gray whale, who was captured in the same lagoon Gigi was from, in 1971, was named Gigi II and was released a year later after becoming too big.[116] The last gray whale, J.J., beached herself in Marina del Rey, California where she was rushed to SeaWorld San Diego and, after 14 months, was released because she got too big to take care of. J.J. was the largest creature to be kept in captivity.[117] The Mito Aquarium in Numazu, Shizuoka (Japan) housed three minke whales in a sea-gate enclosed by nets. One survived for three months, another (a calf) survived for two weeks, and another was kept for a year before breaking through the nets.[118]
References
- ↑ Dictionary.com. "Mysticete". dictionary.reference.com. Retrieved 30 January 2016.
- 1 2 "Mysticeti". Oxford dictionary. Retrieved October 2013.
- 1 2 Bannister 2008, Characteristics and Taxonomy
- ↑ Shorter Oxford English dictionary. United Kingdom: Oxford University Press. 2007. p. 3804. ISBN 0199206872.
- 1 2 Hassanina, Alexandre; Delsucc, Frédéric; Ropiquetd, Anne; Hammere, Catrin; Jansen van Vuurenf, Bettine; Mattheef, Conrad; Ruiz-Garciag, Manuel; Catzeflisc, François; Areskough, Veronika; Thanh Nguyena, Trung; Coulouxj, Arnaud (2012). "Histoire évolutive des Cetartiodactyla (Mammalia, Laurasiatheria) racontée par l’analyse des génomes mitochondriaux". Comptes Rendus Biologies (in French) 335 (1): 32–50. doi:10.1016/j.crvi.2011.11.002.
- 1 2 Sasaki, T.; Nikaido, Masato; Hamilton, Healy; Goto, Mutsuo; Kato, Hidehiro; Kanda, Naohisa; Pastene, Luis; Cao, Ying; Fordyce, R.; Hasegawa, Masami; Okada, Norihiro (2005). "Mitochondrial Phylogenetics and Evolution of Mysticete Whales". Systematic Biology 54 (1): 77–90. doi:10.1080/10635150590905939. PMID 15805012.
- 1 2 Rosenbaum, H. C.; Brownell Jr., R. L.; W.B.C. Schaeff, M.; Portway, V.; White, B. N.; Malik, S.; Pastene, L. A.; J. Patenaude, N.; S. Baker, C.; Goto, M.; Best, P.; Clapham, P. J.; Hamilton, P.; Moore, M.; Payne, R.; Rowntree, V.; T. Tynan, C.; L. Bannister, J.; Desalle, R. (2000). "World-wide genetic differentiation of Eubalaena: Questioning the number of right whale species" (PDF). Molecular Ecology 9 (11): 1793–802. doi:10.1046/j.1365-294x.2000.01066.x. PMID 11091315.
- ↑ Woodward, Becky L.; Winn, Jeremy P.; Fish, Frank E. (2006). "Morphological Specializations of Baleen Whales Associated With Hydrodynamic Performance and Ecological Niche" (PDF). Journal of Morphology 267 (11): 1284–1294. doi:10.1002/jmor.10474. PMID 17051544.
The true buoyancy of a particular whale is dependent upon its body composition, particularly the relative quantities of muscle and blubber tissues. Balaenopterid whales have a higher proportion of muscle tissue and tend to be negatively buoyant while the opposite is true for right whales (Lockyer, 1976).
- ↑ Crane, J.; Scott, R. (2002). "Eubalaena glacialis: North Atlantic right whale: Information". Animal Diversity Web. University of Michigan Museum of Zoology. Retrieved 25 January 2016.
- ↑ Minasian, Stanley M.; Balcomb, Kenneth C.; Foster, Larry, eds. (1984). The World's Whales: The Complete Illustrated Guide. New York: The Smithsonian Institution. p. 18. ISBN 0-89599-014-8.
- ↑ Jones, Mary Lou; L. Swartz, Steven; Leatherwood, Stephen, eds. (1984). "A Review of Gray Whale Feeding Ecology". The Gray Whale: Eschrichtius robustus. pp. 33–34, 423–424. ISBN 978-0123891808.
- ↑ Thomas, Pete (2010). "Gray whale off Israel called 'most amazing sighting in history of whales'". GrindTV.com. Retrieved 25 January 2016.
- ↑ Hoare, Philip (2013). "First grey whale spotted south of the Equator". Retrieved 25 January 2016.
- ↑ Nakamura, G.; Kato, H. (2014). "日本沿岸域に近年(1990–2005 年)出現したコククジラEschrichtius robustus の骨学的特徴,特に頭骨形状から見た北太平洋西部系群と東部系群交流の可能性" (PDF). 哺乳類科学 (in Japanese) 54 (1): 73–88.
- ↑ Arnason, U., Gullberg A. & Widegren, B. (1993). "Cetacean mitochondrial DNA control region: sequences of all extant baleen whales and two sperm whale species". Molecular Biology and Evolution 10 (5): 960–970. PMID 8412655.
- ↑ Potvin, J.; Goldbogen, J. A.; Shadwick, R. E. (2009). "Passive versus active engulfment: verdict from trajectory simulations of lunge-feeding fin whales Balaenoptera physalus". The Royal Society 6 (40): 1005–1025. doi:10.1098/rsif.2008.0492.
- ↑ Dolin, Eric Jay (2007). Leviathan: The History of Whaling in America. W.W. Norton & Co. p. 22. ISBN 0-393-06057-8. Retrieved 25 January 2016.
- ↑ Kemper, Catherine (2008). "Pygmy right whale". In Perrin, W.; Wursig, B.; Thewissen, J. Encyclopedia of Marine Mammals. Academic Press. pp. 939–941. ISBN 0080919936.
- 1 2 E. Fordyce, R.; G. Marx, Felix (2012). "The pygmy right whale Caperea marginata: the last of the cetotheres". The Royal Society 280 (1753). doi:10.1098/rspb.2012.2645.
- 1 2 3 4 5 6 7 G. Marx, Felix (2011). "The More the Merrier? A Large Cladistic Analysis of Mysticetes, and Comments on the Transition from Teeth to Baleen". Journal of Mammalian Evolution 18 (2): 77–100. doi:10.1007/s10914-010-9148-4.
- 1 2 Marx, Felix G.; Tsai, Cheng-Hsiu; Fordyce, R. Ewan (2015). "A new Early Oligocene toothed 'baleen' whale (Mysticeti: Aetiocetidae) from western North America: one of the oldest and the smallest". Royal Society Open Science 2 (12): 150476. doi:10.1098/rsos.150476.
- ↑ Deméré, Berta & McGowen 2005
- ↑ Steeman 2010, Table 1, p. 64
- ↑ G. Marx, Felix; E. Fordyce, R. (2015). "Baleen boom and bust: a synthesis of mysticete phylogeny, diversity and disparity" (PDF). The Royal Society 2. doi:10.1098/rsos.140434.
- ↑ Tsai, Cheng-Hsui; E. Fordyce, R. (2015). "The Earliest Gulp-Feeding Mysticete (Cetacea: Mysticeti) from the Oligocene of New Zealand". Journal of Mammalian Evolution 22 (4): 535–560. doi:10.1007/s10914-015-9290-0.
- ↑ Rose, Kenneth D. (2001). "The Ancestry of Whales" (PDF). Science 293: 2216–2217. doi:10.1126/science.1065305.
- ↑ M.G. Fitzgerald, Erich (2010). "The morphology and systematics of Mammalodon colliveri (Cetacea: Mysticeti), a toothed mysticete from the Oligocene of Australia". Zoological Journal of the Linnean Society 158 (2): 367–476. doi:10.1111/j.1096-3642.2009.00572.x.
- ↑ Deméré, Thomas; Michael R. McGowen; Annalisa Berta; John Gatesy (September 2007). "Morphological and Molecular Evidence for a Stepwise Evolutionary Transition from Teeth to Baleen in Mysticete Whales". Systematic Biology 57 (1): 15–37. doi:10.1080/10635150701884632. Retrieved November 11, 2013.
- 1 2 Uhen 2010, pp. 208–210
- ↑ Fitzgerald 2012, Fig. 2
- ↑ Deméré, Thomas A.; Berta, Annalisa; McGowen, Michael R. (2005). "The taxonomic and evolutionary history of fossil and modern balaenopteroid mysticetes". Journal of Mammalian Evolution 12 (1): 99–143. doi:10.1007/s10914-005-6944-3.
- ↑ E. Steeman, Mette; B. Hebsgaard, Martin; E. Fordyce, R.; Y. W. Ho, Simon; L. Rabosky, Daniel; Nielsen, Rasmus; Rahbek, Carsten; Glenner, Henrik; V. Sørensen, Martin; Willerslev, Eske (2009). "Radiation of Extant Cetaceans Driven by Restructuring of the Oceans". Systematic Biology 58 (6): 573–585. doi:10.1093/sysbio/syp060.
- ↑ Sanders & Barnes 2002
- ↑ Fitzgerald, Erich M. G. (2006). "A bizarre new toothed mysticete (Cetacea) from Australia and the early evolution of baleen whales". Proceedings of the Royal Society 273 (1604): 2955–2963. doi:10.1098/rspb.2006.3664. PMC 1639514. PMID 17015308.
- ↑ P. Dines, James; Otárola-Castillo, Erik; Ralph, Peter; Alas, Jesse; Daley, Timothy; D. Smith, Andrew; D. Dean, Matthew (2014). "Sexual selection targets cetacean pelvic bone". Journal of Organic Evolution 68 (11): 3296–3306. doi:10.1111/evo.12516.
- ↑ Feldhamer 2015, pg. 446.
- 1 2 W. Vogle, A.; A. Lillie, Margo; A. Piscitelli, Marina; A. Goldbogen, Jeremy; D. Pyenson, Nicholas; E. Shadwick, Robert (2015). "Stretchy nerves are an essential component of the extreme feeding mechanism of rorqual whales". Current Biology 25 (9): 360–361. doi:10.1016/j.cub.2015.03.007.
- 1 2 A. Goldbogen, Jeremy (2010). "The Ultimate Mouthful: Lunge Feeding in Rorqual Whales". American Scientist 98 (2): 124. doi:10.1511/2010.83.124.
- 1 2 Welsh, Jennifer (2012). "Whale's Big Gulp Aided by Newfound Organ". Retrieved 23 January 2016.
- ↑ Berta, Annalisa; L. Sumich, James; M. Kovacs, Kit (2015). "Musculoskeletal System and Locomotion". Marine Mammals: Evolutionary Biology (3 ed.). p. 245. ISBN 978-0-12-397002-2.
- ↑ Tinker 1988, p. 66
- 1 2 Cavendish 2010, pg. 101.
- ↑ Cavendish 2010, pg. 95.
- ↑ Washington State Department of Transport. "Estimated Auditory Bandwidths for Marine Mammals and Fish" (PDF). wsdot.wa.gov. Retrieved 24 January 2016.
- ↑ Baraniuk, Chris (2015). "The world's loneliest whale may not be so lonely after all".
- ↑ Cavendish 2010, pg. 96.
- ↑ Yamato, Maya; R. Ketten, Darlene; Arruda, Julie; Cramer, Scott; Moore, Kathleen (2012). "The Auditory Anatomy of the Minke Whale (Balaenoptera acutorostrata): A Potential Fatty Sound Reception Pathway in a Baleen Whale". The Anatomical Record 29 (6): 991–998. doi:10.1002/ar.22459.
- ↑ S. Reidenberg, J.; T. Laitman, J. (2007). "Discovery of a low frequency sound source in Mysticeti (baleen whales): anatomical establishment of a vocal fold homolog". The Anatomical Record 290 (6): 745–759. doi:10.1002/ar.20544. PMID 17516447.
- ↑ Cavendish 2010, pg. 94.
- ↑ Akpan, Nsikan (2014). "Whales Can't Taste Anything But Salt". Retrieved 23 January 2016.
- ↑ J. Ponganis, Paul (2015). "Challenges of the environment". Diving Physiology of Marine Mammals and Seabirds. p. 39. ISBN 978-0-521-76555-8.
- ↑ R. Norena, S.; M. Williams (2000). "Body size and skeletal muscle myoglobin of cetaceans: adaptations for maximizing dive duration". Comparative Biochemistry and Physiology A-molecular & Integrative Physiology 126 (2): 181–191. doi:10.1016/S1095-6433(00)00182-3. PMID 10936758.
- ↑ L. Nelson, D.; M. Cox, M. (2008). Lehninger Principles of Biochemistry (3rd ed.). New York: Worth Publishers. p. 206. ISBN 0-7167-6203-X.
- ↑ Cavendish 2010, pg. 99.
- ↑ Mosbergen, Dominique (2014). "Sleeping Humpback Whale Captured In Rare Footage". Retrieved 23 January 2016.
- ↑ Kellogg, Remington; C. Whitmore, Jr., Frank (1957). "Marine Mammals". Geological Society of America 1 (57): 1223–1224. doi:10.1130/MEM67V1-p1223.
- 1 2 Bannister 2008, pg. 357–361.
- 1 2 J. Aidley, D. (1981). "The Migration of Whales". Animal Migration. p. 111. ISBN 0-521-23274-0.
- ↑ J. Lee, Jane (2015). "A Gray Whale Breaks The Record For Longest Mammal Migration". Retrieved 23 January 2016.
- ↑ G. Sanders, Jon; C. Beichman, Annabel; Roman, Joe; J. Scott, Jarrod; Emerson, David; J. McCarthy, James; R. Girguis, Peter (2015). "Baleen whales host a unique gut microbiome with similarities to both carnivores and herbivores". Nature Communications 6. doi:10.1038/ncomms9285.
- ↑ H. Steele, John (1970). "Feeding pattern of baleen whales in the ocean". Marine Food Chains. pp. 245–247. ISBN 978-0520-01397-1.
- ↑ A. Goldbogen, J.; Calambokidis, J.; Oleson, E.; Potvin, J.; D. Pyenson, N.; Schorr, G.; E. Shadwick, R. (2011). "Mechanics, hydrodynamics and energetics of blue whale lunge feeding: efficiency dependence on krill density". Journal of Experimental Biology 214: 131–146. doi:10.1242/jeb.048157.
- ↑ Kenney, Robert D. (2002). "North Atlantic, North Pacific and Southern Right Whales". In William F. Perrin, Bernd Wursig and J. G. M. Thewissen. The Encyclopedia of Marine Mammals. Academic Press. pp. 806–813. ISBN 0-12-551340-2.
- ↑ H. Ferguson, Steven; W. Higdon, Jeff; H. Westdal, Kristin (2012). "Prey items and predation behavior of killer whales (Orcinus orca) in Nunavut, Canada based on Inuit hunter interviews". Aquatic Biosystems. doi:10.1186/2046-9063-8-3.
- ↑ K. B. Ford, John; R. Reeves, Randall (2008). "Fight or flight: antipredator strategies of baleen whales". Mammal Review 38 (1): 50–86. doi:10.1111/j.1365-2907.2008.00118.x.
- ↑ L. Dicken, M.; A. Kock, A.; Hardenberg, M. (2015). "First observations of dusky sharks (Carcharhinus obscurus) attacking a humpback whale (Megaptera novaeangliae) calf". Marine and Freshwater Research 66 (12): 1211–1215. doi:10.1071/MF14317.
- ↑ Martin, R.A. "Squaliformes Dogfish Sharks". ReefQuest Centre for Shark Research. Retrieved 24 January 2016.
- ↑ Nogata, Yasuyuki; Matsumura, Kiyotaka (2006). "Larval development and settlement of a whale barnacle". Biology Letters 2 (1): 92–93. doi:10.1098/rsbl.2005.0409. PMC 1617185. PMID 17148335.
- ↑ Feldhamer 2015, pg. 457.
- ↑ Bannister 2008, pg. 85.
- ↑ Bannister 2008, Life History
- ↑ Rice, D. W. (1977). "Synopsis of biological data on the sei whale and Bryde's whale in the eastern North Pacific" (PDF). Rep. Int. Whal. Commn. Spec. Iss. 1: 92–97. Retrieved November 2013.
- ↑ Aguilar, A.; Lockyer, C. H. (1987). "Growth, physical maturity, and mortality of fin whales (Balaenoptera physalus) inhabiting the temperate waters of the northeast Atlantic" (PDF). Canadian Journal of Zoology 65 (2): 253–264. doi:10.1139/z87-040. Retrieved November 2013.
- ↑ Ohsumi, S. (1977). "Bryde's whales in the pelagic whaling ground of the North Pacific" (PDF). Rep. Int. Whal. Commn.: 140–9. Retrieved November 2013.
- 1 2 E. Reynolds, J.; A. Rommel, S. (1999). "Reproductive behavior". Biology of marine mammals. ISBN 978-1-58834-250-8.
- ↑ A.R. Knowlton, S.D. Kraus and R.D. Kenney (1994). "Reproduction in North Atlantic right whales (Eubalaena glacialis)". Canadian Journal of Zoology 72 (7): 1297–1305. doi:10.1139/z94-173.
- ↑ H. Duffus, John; M. Templeton, Douglas; Nordberg, Monica (2009). Concepts in Toxicology. p. 171. ISBN 978-0-85404-157-2.
- ↑ Gardner, David (2007). "Whale survives harpoon attack 130 years ago to become 'world's oldest mammal'". Retrieved 6 January 2016.
- ↑ Bannister 2008, Behavior and Physiology
- ↑ Butti, C.; C. Sherwood, C.; Y. Hakeem, A.; M. Allman, J.; R. Hof, P. (2009). "Total number and volume of Von Economo neurons in the cerebral cortex of cetaceans.". The Journal of Comparative Neurology 515 (2): 243–259. doi:10.1002/cne.22055. PMID 19412956.
- ↑ "Rock art hints at whaling origins". 2004. Retrieved 25 January 2016.
Stone Age people may have started hunting whales as early as 6,000 BC, new evidence from South Korea suggests.
- 1 2 3 4 Marrero, Meghan E.; Thornton, Stuart (2011). "Big Fish: A Brief History of Whaling". National Geographic. Retrieved 25 January 2016.
- ↑ Ford, Catherine (2015). "A Savage History: Whaling in the South Pacific and Southern Oceans".
- ↑ P. Proulx, Jean (1994). Basque whaling in Labrador in the 16th century. pp. 260–286. ISBN 978-0-660-14819-9. ISSN 0821-1027.
- ↑ "Whale products". New Bedford Whaling Museum. Retrieved 25 January 2016.
- ↑ Stonehouse, Bernard (2007). "British Arctic whaling: an overview". University of Hull. Retrieved 25 January 2016.
- ↑ Tonnessen, J.N.; Johnsen, A.O (1982). The History of Modern Whaling. C. Hurst. ISBN 0-905838-23-8.
- ↑ R. McNeill, J. (2000). "Whaling and Fishing". An Environmental History of the 20th century. pp. 128–130. ISBN 978-0-393-04917-6.
- ↑ Beckman, Daniel (2013). "Conservation of Cetaceans". Marine Environmental Biology and Conservation. p. 328. ISBN 978-0-7637-7350-2.
- ↑ International Whaling Commission Schedule, para. 10(e).
- ↑ "Keyword search: Baleen whales". The IUCN Red List of Threatened species. Version 2013.1. IUCN. Retrieved 17 July 2013.
- ↑ Elliot, Wendy (2007). Whales in Hot Water? (PDF). World Wildlife Fund. pp. 9–10.
- ↑ Black, Richard (2009). "Whale watching 'worth billions'". Retrieved 27 January 2016.
- ↑ H. Barnes, R. (1996). "Lamakera, Solor. Ethnographic Notes on a Muslim Whaling Village of Eastern Indonesia". Anthropos (91): 75–88.
- ↑ Sfetcu, Nicolae (2011). "Whaling". Fish & Fishing (1 ed.). pp. 169–170. ISBN 978-1-47092935-0.
- 1 2 Beckman, Daniel (2013). "Conservation of Cetaceans". Marine Environmental Biology and Conservation (1 ed.). pp. 327–333. ISBN 978-0-7637-7350-2.
- ↑ Clifford, Frank (1994). "Gray Whale Removed From Endangered List". Retrieved 27 January 2016.
- ↑ White, Doc (2010). "Southern Right Whale". In Fitzpatrick, Lisa. Defying Extinction – Partnerships to Safeguard Global Biodiversity. p. 7. ISBN 978-0-9841686-5-1.
- ↑ Bush Warriors (2011). "IUCN Species of the Day: North Atlantic Right Whale". IUCN. Retrieved 27 January 2016.
- ↑ International Whaling Commission. "Commercial Whaling". iwc.int. Retrieved 30 January 2016.
- ↑ International Whaling Commission. "Scientific Permit Whaling". iwc.int. Retrieved 29 January 2016.
- ↑ H. Schofield, Clive; Lee, Seokwoo; Kwon, Moon-Sang, eds. (2014). "Whaling in the Antarctic: Protecting Rights in Areas Beyond National Jurisdiction Through International Litigation". Limits of Maritime Jurisdiction. p. 527. ISBN 978-90-04-26258-4.
- ↑ Greenpeace International. "Japan and whaling". greenpace.org. Retrieved 29 January 2016.
- ↑ J. Gales, Nicholas; Kasuya, Toshio; J. Clapham, Phillip; L. Brownell, Jr, Robert (2005). "Japan's whaling plan under scrutiny". Nature 435: 883–884. doi:10.1038/435883a.
- ↑ Tabuchi, Hiroko; Simons, Marlise (2014). "U.N. Court Orders Japan to Halt Whaling Off Antarctica". Retrieved 29 January 2016.
- ↑ unknown (2015). "Japan to resume whaling in Antarctic despite court ruling". Retrieved 29 January 2016.
- ↑ S. M. Vanderlaan, Angelia; T. Taggart, Christopher (2007). "Vessel Collisions with Whales: The Probability of Lethal Injury Based on Vessel Speed". Marine Mammal Science 23 (1): 144–156. doi:10.1111/j.1748-7692.2006.00098.x.
- ↑ "Blue Whales Respond to Anthropogenic Noise". PLoS ONE 7: e32681. Bibcode:2012PLoSO...732681M. doi:10.1371/journal.pone.0032681. PMC 3290562. PMID 22393434.
- ↑ R. Reeves, R.; Clapham, PJ.; L. Brownell, R.; G., K. Silber (1998). Recovery plan for the blue whale (Balaenoptera musculus) (PDF). National Marine Fisheries Service. p. 42.
- ↑ "Blue Whales Respond to Anthropogenic Noise". PLoS ONE 7: e32681. Bibcode:2012PLoSO...732681M. doi:10.1371/journal.pone.0032681. PMC 3290562. PMID 22393434.
- ↑ J. O'Shea, Thomas; L. Brownell, Jr., Robert (1994). "Organochlorine and metal contaminants in baleen whales: a review and evaluation of conservation implications". Science of the Total Environment 154 (3): 179–200. doi:10.1016/0048-9697(94)90087-6.
- ↑ Office of Protected Resources – NOAA Fisheries. "North Atlantic Right Whale (Eubalaena glacialis)". nmfs.noaa.gov. Retrieved 25 January 2016.
- ↑ NOAA Marine Debris Report (2014). "Cetaceans". Entanglement of Marine Species in Marine Debris with an Emphasis on Species in the United States (PDF). pp. 9–10.
- ↑ Perry, Tony (1998). "J.J. The Gray Whale Going To Sea -- Rescued Orphan Calf Will Be Freed This Week". Retrieved 29 January 2016.
- ↑ L. Hubbs, Carl; E. Evans, William (1974). "The California gray whale : papers presented at the California Gray Whale Workshop, Scripps Institution of Oceanography". Marine Fisheries Review 36 (4).
- ↑ L. Sumich, J.; Goff, T.; L. Perryman, W. (2001). "Growth of two captive gray whale calves" (PDF). Aquatic Mammals 27 (3): 231–233.
- ↑ Perry, Tony (1998). "Rescued Whale J.J. Begins Long Journey Home". Retrieved 29 January 2016.
- ↑ Kimura, S.; Nemoto, T. (1956). "Note on a minke whale kept alive in aquarium". Scientific Reports of the Whales Research Institute 11: 181–189.
Further reading
- Kemper, Catherine (2008). Perrin, W.; Wursig, B.; Thewissen, J., eds. Encyclopedia of Marine Mammals. Academic Press. ISBN 0080919936.
- Cavendish, Marshall (2010). "Gray whale". Mammal Anatomy: An Illustrated Guide. ISBN 978-0-7614-7882-9.
- Uhen, M. D. (2010). "The origin(s) of whales". Annual Review of Earth and Planetary Sciences 38: 189–219. doi:10.1146/annurev-earth-040809-152453.
- W. Tinker, Spencer (1988). Whales of the World. Brill Archive. ISBN 9780935848472. Retrieved October 2013.
- L. Bannister, John (2008). "Baleen Whales (Mysticetes)". In F. Perrin, William; Würsig, Bernd; Thewissen, J. G. M. Encyclopedia of Marine Mammals (2 ed.). Academic Press. pp. 80–89. ISBN 978-0-12-373553-9.
- A. Deméré, T.; Berta, A.; R. McGowen, M. (2005). "The taxonomic and evolutionary history of fossil and modern balaenopteroid mysticetes". Journal of Mammalian Evolution 12 (1): 99–143. doi:10.1007/s10914-005-6944-3. OCLC 264019292.
- W. Rice, Dale (1998). "Marine mammals of the world: systematics and distribution". Society for Marine Mammalogy: 1–231. OCLC 40622084.
- A. Feldhamer, George; C. Drickamer, Lee; H. Vessey, Stephen; F. Merritt, Joseph; Krajewski, Carey (2015). "Cetacea". Mammalogy: Adaptation, Diversity, Ecology. ISBN 978-1-4214-1588-8.
| Wikimedia Commons has media related to Mysticeti. |