MutS-1
| MutS_I | |||||||||
|---|---|---|---|---|---|---|---|---|---|
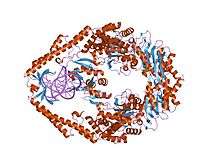 The crystal structure of E. coli MutS binding to DNA with an a:a mismatch | |||||||||
| Identifiers | |||||||||
| Symbol | MutS_I | ||||||||
| Pfam | PF01624 | ||||||||
| InterPro | IPR007695 | ||||||||
| SMART | MUTSd | ||||||||
| PROSITE | PDOC00388 | ||||||||
| SCOP | 1ng9 | ||||||||
| SUPERFAMILY | 1ng9 | ||||||||
| |||||||||
Mismatch repair contributes to the overall fidelity of DNA replication and is essential for combating the adverse effects of damage to the genome. It involves the correction of mismatched base pairs that have been missed by the proofreading element (Klenow fragment) of the DNA polymerase complex. The post-replicative Mismatch Repair System (MMRS) of Escherichia coli involves MutS (Mutator S), MutL and MutH proteins, and acts to correct point mutations or small insertion/deletion loops produced during DNA replication.[1] MutS and MutL are involved in preventing recombination between partially homologous DNA sequences. The assembly of MMRS is initiated by MutS, which recognizes and binds to mispaired nucleotides and allows further action of MutL and MutH to eliminate a portion of newly synthesized DNA strand containing the mispaired base.[2] MutS can also collaborate with methyltransferases in the repair of O(6)-methylguanine damage, which would otherwise pair with thymine during replication to create an O(6)mG:T mismatch.[3] MutS exists as a dimer, where the two monomers have different conformations and form a heterodimer at the structural level.[4] Only one monomer recognises the mismatch specifically and has ADP bound. Non-specific major groove DNA-binding domains from both monomers embrace the DNA in a clamp-like structure. Mismatch binding induces ATP uptake and a conformational change in the MutS protein, resulting in a clamp that translocates on DNA.
MutS is a modular protein with a complex structure,[5] and is composed of:
- N-terminal mismatch-recognition domain, which is similar in structure to tRNA endonuclease.
- Connector domain, which is similar in structure to Holliday junction resolvase ruvC.
- Core domain, which is composed of two separate subdomains that join together to form a helical bundle; from within the core domain, two helices act as levers that extend towards (but do not touch) the DNA.
- Clamp domain, which is inserted between the two subdomains of the core domain at the top of the lever helices; the clamp domain has a beta-sheet structure.
- ATPase domain (connected to the core domain), which has a classical Walker A motif.
- HTH (helix-turn-helix) domain, which is involved in dimer contacts.
Homologues of MutS have been found in many species including eukaryotes (MSH 1, 2, 3, 4, 5, and 6 proteins), archaea and bacteria, and together these proteins have been grouped into the MutS family. Although many of these proteins have similar activities to the E. coli MutS, there is significant diversity of function among the MutS family members. This diversity is even seen within species, where many species encode multiple MutS homologues with distinct functions.[6] Inter-species homologues may have arisen through frequent ancient horizontal gene transfer of MutS (and MutL) from bacteria to archaea and eukaryotes via endosymbiotic ancestors of mitochondria and chloroplasts.[7]
This entry represents the N-terminal domain of proteins in the MutS family of DNA mismatch repair proteins, as well as closely related proteins. The N-terminal domain of MutS is responsible for mismatch recognition and forms a 6-stranded mixed beta-sheet surrounded by three alpha-helices, which is similar to the structure of tRNA endonuclease. Yeast MSH3,[8] bacterial proteins involved in DNA mismatch repair, and the predicted protein product of the Rep-3 gene of mouse share extensive sequence similarity. Human MSH has been implicated in non-polyposis colorectal carcinoma (HNPCC) and is a mismatch binding protein.
References
- ↑ Nag N, Rao BJ, Krishnamoorthy G (November 2007). "Altered dynamics of DNA bases adjacent to a mismatch: a cue for mismatch recognition by MutS". J. Mol. Biol. 374 (1): 39–53. doi:10.1016/j.jmb.2007.08.065. PMID 17919654.
- ↑ Miguel V, Pezza RJ, Argaraña CE (August 2007). "The C-terminal region of Escherichia coli MutS and protein oligomerization". Biochem. Biophys. Res. Commun. 360 (2): 412–7. doi:10.1016/j.bbrc.2007.06.056. PMID 17599803.
- ↑ Rye PT, Delaney JC, Netirojjanakul C, Sun DX, Liu JZ, Essigmann JM (February 2008). "Mismatch repair proteins collaborate with methyltransferases in the repair of O(6)-methylguanine". DNA Repair (Amst.) 7 (2): 170–6. doi:10.1016/j.dnarep.2007.09.003. PMC 3015234. PMID 17951114.
- ↑ Mendillo ML, Putnam CD, Kolodner RD (June 2007). "Escherichia coli MutS tetramerization domain structure reveals that stable dimers but not tetramers are essential for DNA mismatch repair in vivo". J. Biol. Chem. 282 (22): 16345–54. doi:10.1074/jbc.M700858200. PMID 17426027.
- ↑ Lamers MH, Perrakis A, Enzlin JH, Winterwerp HH, de Wind N, Sixma TK (October 2000). "The crystal structure of DNA mismatch repair protein MutS binding to a G x T mismatch". Nature 407 (6805): 711–7. doi:10.1038/35037523. PMID 11048711.
- ↑ Eisen JA (September 1998). "A phylogenomic study of the MutS family of proteins". Nucleic Acids Res. 26 (18): 4291–300. doi:10.1093/nar/26.18.4291. PMC 147835. PMID 9722651.
- ↑ Lin Z, Nei M, Ma H (2007). "The origins and early evolution of DNA mismatch repair genes--multiple horizontal gene transfers and co-evolution". Nucleic Acids Res. 35 (22): 7591–603. doi:10.1093/nar/gkm921. PMC 2190696. PMID 17965091.
- ↑ New L, Liu K, Crouse GF (May 1993). "The yeast gene MSH3 defines a new class of eukaryotic MutS homologues". Mol. Gen. Genet. 239 (1-2): 97–108. doi:10.1007/BF00281607. PMID 8510668.
This article incorporates text from the public domain Pfam and InterPro IPR007695