Mukaiyama aldol addition
| educts |
|---|
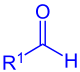 aldehyde (R1 = Alkyl, Aryl) or formate (R1 = OR) |
 silyl enol ether (R1 = Alkyl, Aryl, H; R2 = Alkyl, Aryl, H, OR, SR) |
The Mukaiyama aldol addition is an organic reaction and a type of aldol reaction between a silyl enol ether and an aldehyde or formate.[1] The reaction was discovered by Teruaki Mukaiyama (* 1927) in 1973.[2] His choice of reactants allows for a crossed aldol reaction between an aldehyde and a ketone or a different aldehyde without self-condensation of the aldehyde. For this reason the reaction is used extensively in organic synthesis.
General Reaction Scheme
The Mukaiyama aldol addition is a Lewis acid mediated addition of enol silanes to carbonyl compounds. In this reaction compounds with various organic groups can be used (see educts).[3]
A basic version (R2 = H) without the presence of chiral catalysts is shown below.
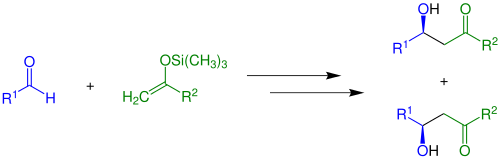
A racemic mix of enantiomers is built. If Z- or E-enol silanes are used in this reaction a mixture of four products occurs, yielding two racemates.
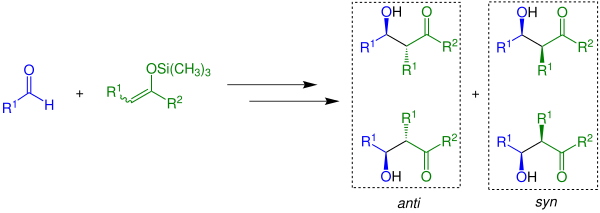
If the anti-diastereomer or the syn-diastereomer is built largely depends on reaction conditions, substrates and Lewis acids.
The archetypical reaction is that of the silyl enol ether of cyclohexanone with benzaldehyde. At room temperature it produces a diastereomeric mixture of threo (63%) and erythro (19%) β-hydroxyketone as well as 6% of the exocyclic enone condensation product. In its original scope the Lewis acid (titanium chloride) was used in stoichiometric amounts but truly catalytic systems exist as well. The reaction is also optimized for asymmetric synthesis.
Mechanism
Below, the reaction mechanism is shown with R2 = H:

In the cited example the Lewis acid TiCl4 is used. First, the Lewis acid activates the aldehyde component followed by carbon-carbon bond formation between the enol silane and the activated aldehyde. With the loss of a chlorosilane the compound 1 is built. The desired product, a racemate of 2 and 3, is obtained by aqueous work-up.[3]
Scope
A typical reaction involving two ketones is that between acetophenone as the enol and acetone:[4]
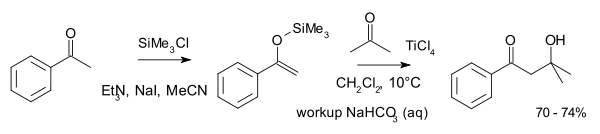
Ketone reactions of this type require higher reaction temperatures. For this work Mukaiyama was inspired by earlier work done by Georg Wittig in 1966 on crossed aldol reactions with lithiated imines.[5][6] Competing work with lithium enolate aldol reactions was published also in 1973 by Herbert O. House.[7]
Mukaiyama employed in his rendition of taxol total synthesis (1999) two aldol additions,[8][9] one with an ketene silyl acetal and excess magnesium bromide:
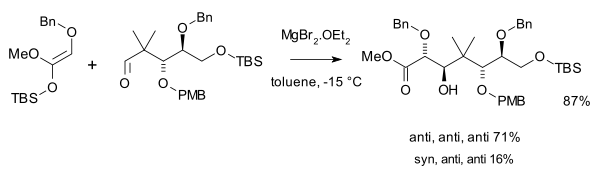
and a second one with an amine chiral ligand and a triflate salt catalyst:
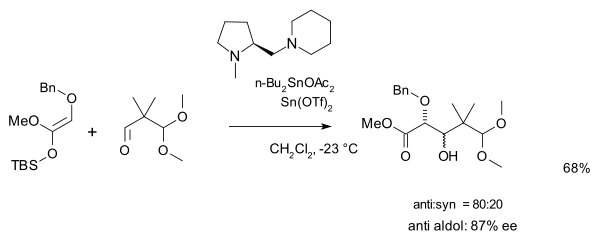
Utilization of chiral Lewis acid complexes and Lewis bases in asymmetric catalytic processes is the fastest-growing area in the usage of the Mukaiyama aldol reaction.[3]
References
- ↑ Mukaiyama, T.; Kobayashi, S. Org. React. 1994, 46, 1. doi:10.1002/0471264180.or046.01
- ↑ New aldol type reaction Teruaki Mukaiyama, Koichi Narasaka and Kazuo Banno Chemistry Letters Vol.2 (1973) , No.9 pp.1011–1014 doi:10.1246/cl.1973.1011
- 1 2 3 László Kürti und Barbara Czakó.: Strategic Applications of Named Reactions in Organic Synthesis: Background and Detailed Mechanisms, Elsevier Academic Press, 2005, S. 298–299, ISBN 978-0-12-429785-2.
- ↑ Organic Syntheses, Coll. Vol. 8, p.323 (1993); Vol. 65, p. 6 (1987). http://www.orgsynth.org/orgsyn/pdfs/CV8P0323.pdf
- ↑ Über gezielte Aldolkondensationen—II G. Wittig and P. Suchanek Tetrahedron Volume 22, Supplement 8, 1966, Pages 347–358 doi:10.1016/S0040-4020(01)82193-1
- ↑ DIRECTED ALDOL CONDENSATIONS: β-PHENYLCINNAMALDEHYDE Organic Syntheses, Coll. Vol. 6, p.901 (1988); Vol. 50, p.66 (1970). G. Wittig, A. Hesse, Allan Y. Teranishi and Herbert O. House http://www.orgsynth.org/orgsyn/prep.asp?prep=cv6p0901
- ↑ Chemistry of carbanions. XXIII. Use of metal complexes to control the aldol condensation Herbert O. House, David S. Crumrine, Allan Y. Teranishi, Hugh D. Olmstead J. Am. Chem. Soc.; 1973; 95(10); 3310–24. doi:10.1021/ja00791a039
- ↑ Asymmetric Total Synthesis of Taxol Teruaki Mukaiyama , Isamu Shiina, Hayato Iwadare, Masahiro Saitoh, Toshihiro Nishimura, Naoto Ohkawa, Hiroki Sakoh, Koji Nishimura, Yu-ichirou Tani, Masatoshi Hasegawa, Koji Yamada , Katsuyuki Saitoh Chem. Eur. J. 1999, 5, No. 1 doi:10.1002/(SICI)1521-3765(19990104)5:1<121::AID-CHEM121>3.0.CO;2-O
- ↑ TBS = t-butyldimethylsilyl, Bn = benzyl, PMB = p-methoxybenzyl ether