Mubritinib
 | |
| Systematic (IUPAC) name | |
|---|---|
|
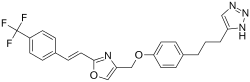
1-(4-{4-[(2-{(E)-2-[4-(trifluoromethyl)phenyl]ethenyl}-1,3-oxazol-4-yl)methoxy]phenyl}butyl)-1H-1,2,3-triazole | |
| Clinical data | |
| Legal status |
|
| Routes of administration | Oral |
| Identifiers | |
| CAS Number | 366017-09-6 |
| ATC code | None |
| PubChem | CID 6444692 |
| IUPHAR/BPS | 6011 |
| ChemSpider | 4948554 |
| UNII |
V734AZP9BR |
| ChEMBL | CHEMBL1614707 |
| Chemical data | |
| Formula | C25H23F3N4O2 |
| Molar mass | 468.47 g/mol |
| |
Mubritinib (TAK-165) is a protein kinase inhibitor which was under development by Takeda for the treatment of cancer.[1][2][3] It completed phase I clinical trials but appears to have been discontinued, as no new information on the drug has surfaced since December 2008.[4]
Synthesis

See also
References
- ↑ McCormick, Frank; Doriano Fabbro (2005). Protein Tyrosine Kinases: From Inhibitors to Useful Drugs (Cancer Drug Discovery and Development). Totowa, NJ: Humana Press. ISBN 1-58829-384-X.
- ↑ Mitscher, Lester A.; Lednicer, Daniel (1977). The organic chemistry of drug synthesis. New York: Wiley. ISBN 0-470-10750-2.
- ↑ Lednicer, Daniel (2008). Strategies for Organic Drug Synthesis and Design. New York: Wiley-Interscience. ISBN 0-470-19039-6.
- ↑ "Safety and Tolerability Study of TAK-165 in Subjects With Tumors Expressing HER2 - Full Text View - ClinicalTrials.gov".
| ||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||
This article is issued from Wikipedia - version of the Tuesday, June 16, 2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.