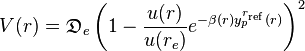Morse/Long-range potential
| Computational physics |
|---|
 |
|
Numerical analysis · Simulation |
|
Particle |
|
Scientists |
Owing to the simplicity of the Morse potential (it only has three adjustable parameters), it is not used in modern spectroscopy. The MLR (Morse/Long-range) potential is a modern version of the Morse potential which has the correct theoretical long-range form of the potential naturally built into it.[1] It was first introduced by professor Robert J. Le Roy of University of Waterloo, professor Nikesh S. Dattani of Oxford University and professor John A. Coxon of Dalhousie University in 2009[1] Since then it has been an important tool for spectroscopists to represent experimental data, verify measurements, and make predictions. It is particularly renowned for its extrapolation capability when data for certain regions of the potential are missing, its ability to predict energies with accuracy often better than the most sophisticated ab initio techniques, and its ability to determine precise empirical values for physical parameters such as the dissociation energy, equilibrium bond length, and long-range constants. Cases of particular note include:
- the c-state of Li2: where the MLR potential was successfully able to bridge a gap of more than 5000 cm−1 in experimental data.[2] Two years later it was found that Dattani's MLR potential was able to successfully predict the energies in the middle of this gap, correctly within about 1 cm−1.[3] The accuracy of these predictions was much better than the most sophisticated ab initio techniques at the time.[4]
- the A-state of Li2: where Le Roy et al.[1] constructed an MLR potential which determined the C3 value for atomic lithium to a higher-precision than any previously measured atomic oscillator strength, by an order of magnitude.[5] This lithium oscillator strength is related to the radiative lifetime of atomic lithium and is used as a benchmark for atomic clocks and measurements of fundamental constants. It has been said that this work by Le Roy et al. was a "landmark in diatomic spectral analysis".[5]
- the a-state of KLi: where an analytic global (MLR) potential was successfully built despite there only being a small amount of data near the top of the potential.[6]
Historical origins
The MLR potential is based on the classic Morse potential which was first introduced in 1929 by Philip M. Morse. A primitive version of the MLR potential was first introduced in 2006 by professor Robert J. Le Roy and colleagues for a study on N2.[7] This primitive form was used on Ca2,[8] KLi[6] and MgH,[9][10][11] before the more modern version was introduced in 2009 by Le Roy, Dattani, and Coxon.[1] A further extension of the MLR potential referred to as the MLR3 potential was introduced in a 2010 study of Cs2,[12] and this potential has since been used on HF,[13][14] HCl,[13][14] HBr[13][14] and HI.[13][14]
Function
The Morse/Long-range potential energy function is of the form
where for large 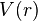 ,
,
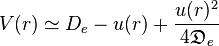 ,
,
so 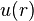 is defined according to the theoretically correct long-range behavior expected for the interatomic interation.
is defined according to the theoretically correct long-range behavior expected for the interatomic interation.
This long-range form of the MLR model is guaranteed because the argument of the exponent is defined to have long-range behavior:
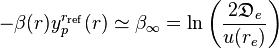 ,
,
There are a few ways in which this long-range behavior can be achieved, the most common is to make 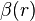 a polynomial that is constrained to become
a polynomial that is constrained to become 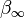 at long-range:
at long-range:
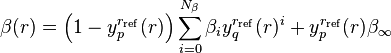 ,
,
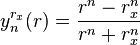 .
.
It is clear to see that:
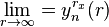 , so
, so
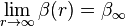 .
.
Applications
The MLR potential has successfully summarized all experimental spectroscopic data (and/or virial data) for a number of diatomic molecules, including: N2,[7] Ca2,[8] KLi,[6] MgH,[9][10][11] several electronic states of Li2,[1][2][15][3][10] Cs2,[16][12] Sr2,[17] ArXe,[10][18] LiCa,[19] LiNa,[20] Br2,[21] Mg2,[22] HF,[13][14] HCl,[13][14] HBr,[13][14] HI,[13][14] MgD,[9] Be2,[23] BeH,[24] and NaH.[25] More sophisticated versions are used for polyatomic molecules.
It has also become customary to fit ab initio points to the MLR potential, to achieve a fully analytic ab initio potential and to take advantage of the MLR's ability to incorporate the correct theoretically known short- and long-range behavior into the potential (the latter usually being of higher accuracy than the molecular ab initio points themselves because it is based on atomic ab initio calculations rather than molecular ones, and because features like spin-orbit coupling which are difficult to incorporate into molecular ab inito calculations can more easily be treated described in the long-range). Examples of molecules for which the MLR has been used to represent ab initio points are KLi,[26] KBe.[27]
See also
References
- 1 2 3 4 5 Le Roy, Robert J.; N. S. Dattani; J. A. Coxon; A. J. Ross; Patrick Crozet; C. Linton (2009). "Accurate analytic potentials for Li2(X) and Li2(A) from 2 to 90 Angstroms, and the radiative lifetime of Li(2p)". Journal of Chemical Physics 131 (20): 204309. doi:10.1063/1.3264688.
- 1 2 Dattani, N. S.; R. J. Le Roy (8 May 2013). "A DPF data analysis yields accurate analytic potentials for Li2(a) and Li2(c) that incorporate 3-state mixing near the c-state asymptote". Journal of Molecular Spectroscopy (Special Issue) 268: 199–210. doi:10.1016/j.jms.2011.03.030.
- 1 2 Semczuk, M.; Li, X.; Gunton, W.; Haw, M.; Dattani, N. S.; Witz, J.; Mills, A. K.; Jones, D. J.; Madison, K. W. (2013). "High-resolution photoassociation spectroscopy of the 6Li2 13Σ+ state". Phys. Rev. A 87. p. 052505. doi:10.1103/PhysRevA.87.052505.
- ↑ Halls, M. S.; H. B. Schlegal; M. J. DeWitt; G. F. W. Drake (18 May 2001). "Ab initio calculation of the a-state interaction potential and vibrational levels of 7Li2" (PDF). Chemical Physics Letters 339 (5–6): 427–432. doi:10.1016/s0009-2614(01)00403-1.
- 1 2 Tang, Li-Yan; Z-C. Yan, T-Y Shi, J. Mitroy; Shi, Ting-Yun; Mitroy, J. (30 November 2011). "Third-order perturbation theory for van der Waals interaction coefficients". Physical Review A 84 (5): 052502. doi:10.1103/PhysRevA.84.052502.
- 1 2 3 Salami, H.; A. J. Ross; P. Crozet; W. Jastrzebski; P. Kowalczyk; R. J. Le Roy (2007). "A full analytic potential energy curve for the a3Σ+ state of KLi from a limited vibrational data set". Journal of Chemical Physics 126 (19): 194313. doi:10.1063/1.2734973.
- 1 2 Le Roy, R. J.; Y. Huang; C. Jary (2006). "An accurate analytic potential function for ground-state N2 from a direct-potential-fit analysis of spectroscopic data". Journal of Chemical Physics 125 (16): 164310. doi:10.1063/1.2354502.
- 1 2 Le Roy, Robert J.; R. D. E. Henderson (2007). "A new potential function form incorporating extended long-range behaviour: application to ground-state Ca2". Molecular Physics 105 (5–7): 663–677. doi:10.1080/00268970701241656.
- 1 2 3 Henderson, R. D. E.; A. Shayesteh; J. Tao; C. Haugen; P. F. Bernath; R. J. Le Roy (4 October 2013). "Accurate Analytic Potential and Born–Oppenheimer Breakdown Functions for MgH and MgD from a Direct-Potential-Fit Data Analysis". The Journal of Physical Chemistry A 117 (50): 131028105904004. doi:10.1021/jp406680r.
- 1 2 3 4 Le Roy, R. J.; C. C. Haugen; J. Tao; H. Li (February 2011). "Long-range damping functions improve the short-range behaviour of 'MLR' potential energy functions" (PDF). Molecular Physics 109 (3): 435–446. doi:10.1080/00268976.2010.527304.
- 1 2 Shayesteh, A.; R. D. E. Henderson; R. J. Le Roy; P. F. Bernath (2007). "Ground State Potential Energy Curve and Dissociation Energy of MgH". The Journal of Physical Chemistry A 111 (49): 12495–12505. doi:10.1021/jp075704a. PMID 18020428.
- 1 2 Coxon, J. A.; P. G. Hajigeorgiou (2010). "The ground X 1Σ+g electronic state of the cesium dimer: Application of a direct potential fitting procedure". Journal of Chemical Physics 132 (9): 094105. doi:10.1063/1.3319739.
- 1 2 3 4 5 6 7 8 Li, Gang; I. E. Gordon; P. G. Hajigeorgiou; J. A. Coxon; L. S. Rothman (2013). "Reference spectroscopic data for hydrogen halides, Part II: The line lists". Journal of Quantitative Spectroscopy & Radiative Transfer 130: 284–295. Bibcode:2013JQSRT.130..284L. doi:10.1016/j.jqsrt.2013.07.019.
- 1 2 3 4 5 6 7 8 Coxon, John A.; Hajigeorgiou, Photos G. (2015). "Improved direct potential fit analyses for the ground electronic states of the hydrogen halides: HF/DF/TF, HCl/DCl/TCl, HBr/DBr/TBr and HI/DI/TI". Journal of Quantitative Spectroscopy and Radiative Transfer 151: 133. doi:10.1016/j.jqsrt.2014.08.028.
- ↑ Gunton, Will; Semczuk, Mariusz; Dattani, Nikesh S.; Madison, Kirk W. (2013). "High resolution photoassociation spectroscopy of the 6Li2 A(11Σu+) state". Physical Review A 88 (6). arXiv:1309.5870. doi:10.1103/PhysRevA.88.062510.
- ↑ Xie, F.; L. Li; D. Li; V. B. Sovkov; K. V. Minaev; V. S. Ivanov; A. M. Lyyra; S. Magnier (2011). "Joint analysis of the Cs2 a-state and 1g(33Π11g) states". Journal of Chemical Physics 135 (2): 02403. doi:10.1063/1.3606397.
- ↑ Stein, A.; H. Knockel; E. Tiemann (April 2010). "The 1S+1S asymptote of Sr2 studied by Fourier-transform spectroscopy". The European Physical Journal D 57 (2): 171–177. doi:10.1140/epjd/e2010-00058-y.
- ↑ Piticco, Lorena; F. Merkt; A. A. Cholewinski; F. R. W. McCourt; R. J. Le Roy (December 2010). "Rovibrational structure and potential energy function of the ground electronic state of ArXe". Journal of Molecular Spectroscopy 264 (2): 83–93. doi:10.1016/j.jms.2010.08.007.
- ↑ Ivanova, Milena; A. Stein; A. Pashov; A. V. Stolyarov; H. Knockel; E. Tiemann (2011). "The X2Σ+ state of LiCa studied by Fourier-transform spectroscopy". Journal of Chemical Physics 135 (17): 174303. doi:10.1063/1.3652755.
- ↑ Steinke, M.; H. Knockel; E. Tiemann (27 April 2012). "X-state of LiNa studied by Fourier-transform spectroscopy". Physical Review A 85 (4): 042720. doi:10.1103/PhysRevA.85.042720.
- ↑ Yukiya, T.; N. Nishimiya; Y. Samejima; K. Yamaguchi; M. Suzuki; C. D. Boonec; I. Ozier; R. J. Le Roy (January 2013). "Direct-potential-fit analysis for the system of Br2". Journal of Molecular Spectroscopy 283: 32–43. doi:10.1016/j.jms.2012.12.006.
- ↑ Knockel, H.; S. Ruhmann; E. Tiemann (2013). "The X-state of Mg2 studied by Fourier-transform spectroscopy". Journal of Chemical Physics 138 (9): 094303. Bibcode:2013JChPh.138i4303K. doi:10.1063/1.4792725.
- ↑ "Direct-potential-fit analyses yield improved empirical potentials for the ground X1Σg+ state of Be2". doi:10.1063/1.4864355.
- ↑ Dattani, Nikesh S. (2015). "Beryllium monohydride (BeH): Where we are now, after 86years of spectroscopy". Journal of Molecular Spectroscopy 311: 76–83. doi:10.1016/j.jms.2014.09.005.
- ↑ "Dissociation energies and potential energy functions for the ground X 1Σ+ and "avoided-crossing" A 1Σ+ states of NaH". doi:10.1063/1.4906086.
- ↑ "The effect of inner-shell electrons on the ground and low-lying excited states of KLi: Ab initio study with all-electron basis sets". doi:10.1016/j.jqsrt.2013.05.025.
- ↑ "An ab initio study of the ground and low-lying excited states of KBe with the effect of inner-shell electrons". doi:10.1063/1.4818452.