Morin (flavonol)
 | |
 | |
| Names | |
|---|---|
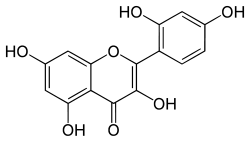
| IUPAC name
2-(2,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one | |
| Other names
Aurantica Al-Morin Morin hydrate Calico Yellow Toxylon pomiferum Bois d'arc Osage orange extract | |
| Identifiers | |
| 654055-01-3 | |
| ChEBI | CHEBI:75092 |
| ChEMBL | ChEMBL28626 |
| ChemSpider | 4444989 |
| 411 | |
| Jmol interactive 3D | Image |
| KEGG | C10105 |
| PubChem | 5281670 |
| |
| |
| Properties | |
| C15H10O7 | |
| Molar mass | 302.2357 g/mol |
| Density | 1.799 g/mL |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Morin is a chemical compound. It is a yellow color substance that can be isolated from Maclura pomifera (Osage orange), Maclura tinctoria (old fustic) and from leaves of Psidium guajava (common guava).[1] In a preclinical in vitro study, morin was found to be a weak inhibitor of fatty acid synthase with an IC50 of 2.33 μM.[2]
Morin can be used to test for the presence of aluminium or tin in a solution, since it forms characteristically fluorescent coordination complexes with them.
Glycosides
- Morin-3-O-arabinoside[1]
- Morin-3-O-lyxoside[1]
References
- 1 2 3 Bacteriostatic effect of flavonoids isolated from leaves of Psidium guajava on fish pathogens. Rattanachaikunsopon Pongsak and Phumkhachorn Parichat, INIST:19087798
- ↑ Tian, WX (February 2006). "Inhibition of Fatty Acid Synthase by Polyphenols". Current Medicinal Chemistry 13 (8): 967–977. doi:10.2174/092986706776361012. PMID 16611078.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This article is issued from Wikipedia - version of the Monday, November 02, 2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.