Montréalone
A montréalone (synonyms: montrealone, phospha-münchnone) is a mesoionic heterocyclic chemical compound.
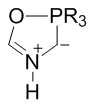
Structure
Montréalones are a class of unsaturated 5-membered organophosphorus heterocycle which incorporate within their ring system overlapping azomethine ylide and Wittig-type moieties.[1][2] Depending on the phosphorus substituents, ring-chain valence tautomerism allows montréalones to display variable degrees of equilibrium and structural blending with N-acylamino phosphonium ylide forms. The cyclic 1,3-dipolar form is detectably or exclusively formed with phosphite and phosphonite derivatives. Triphenylphosphine derivatives lack the capacity for cyclization, and exist as acylic phosphonium ylides.[3]

Synthesis
Montréalones represent a special case of Wittig reagents and are generated analogously. Reaction of organophosphorus(III) compounds with N-acyliminium ions affords phosphonium salt intermediates which are deprotonated using non-nucleophilic bases (e.g. DBU, LiHMDS). As the N-acyliminium ions are generated in situ from acid chlorides and imines, '1,3-dipole'-generation is a multicomponent process. The most efficient phosphorus(III) precursor is 2-phenylbenzo[d][1,3,2]dioxaphosphole [PhP(catechyl)] owing to the nucleophilicity/electrophilicity balance it affords.[2]

Reactions
Montréalones are efficient 1,3-dipolar cycloaddition reagents in one-pot reactions involving dipolarophiles such as imines, alkenes, and alkynes to respectively afford imidazoles,[4] 2-pyrrolines,[5] and pyrroles.[6][7]

Cycloaddition reactions of asymmetric 1,3-dipoles and dipolarophiles can lead to isomeric product mixtures, particularly with münchnones and alkynes in the synthesis of pyrroles.[8][9][10] In contrast to related Diels-Alder reactions, rationalization of regioisomeric bias using conventional frontier molecular orbital (FMO) theory fails. The complimentary use of montéalones and münchnones allows product mixtures to be avoided and highlights the need to consider transition-state geometrical changes (distortion) in the rationalization process.[11]

See also
References
- ↑ Reissig, H.-U.; Zimmer, R., Münchnones—New Facets after 50 Years. Angew. Chem. Int. Edit. 2014, 53, 9708. (doi:10.1002/anie.201405092)
- ↑ 2.0 2.1 St. Cyr, D. J.; Arndtsen, B. A., A New Use of Wittig-type Reagents as 1,3-Dipolar Cycloaddition Precursors and in Pyrrole Synthesis. J. Am. Chem. Soc. 2007, 129, 12366. (doi:10.1021/ja074330w)
- ↑ Krenske, E. H.; Houk, K. N.; Arndtsen, B. A.; St. Cyr, D. J., Cyclic 1,3-Dipoles or Acyclic Phosphonium Ylides? Electronic Characterization of "Montréalones". J. Am. Chem. Soc. 2008, 130, 10052. (doi:10.1021/ja802646f)
- ↑ Aly, S.; Romashko, M.; Arndtsen, B. A., Multicomponent Synthesis of Substituted and Fused-Ring Imidazoles via Phospha-münchnone Cycloaddition. J. Org. Chem. 2015, 80, 2709. (doi:10.1021/jo5028936)
- ↑ Morin, M. S. T.; Arndtsen, B. A., Chiral Phosphorus-Based 1,3-Dipoles: A Modular Approach to Enantioselective 1,3-Dipolar Cycloaddition and Polycyclic 2-Pyrroline Synthesis. Org. Lett. 2014, 16, 1056. (doi:10.1021/ol4035512)
- ↑ St-Cyr, D. J.; Morin, M. S. T.; Belanger-Gariepy, F.; Arndtsen, B. A.; Krenske, E. H.; Houk, K. N., Phospha-Münchnones: Electronic Structures and 1,3-Dipolar Cycloadditions. J. Org. Chem. 2010, 75, 4261. (doi:10.1021/jo1008383)
- ↑ Organic Chemistry Portal pyrrole synthesis abstract: "A New Use of Wittig-Type Reagents as 1,3-Dipolar Cycloaddition Precursors and in Pyrrole Synthesis.". Retrieved 6 April 2015.
- ↑ Lubell, W.; St-Cyr, D.; Dufour-Gallant, J.; Hopewell, R.; Boutard, N.; Kassem, T.; Dörr, A.; Zelli, R., "1H-Pyrroles (Update 2013)". Science of Synthesis 2013, 2013/1, 157-388.
- ↑ Gribble, G. W. In Oxazoles: Synthesis, Reactions, and Spectroscopy, A; Palmer, D. C., Ed.; Wiley: New York, 2003; Vol. 60. (doi:10.1002/0471428035.ch4)
- ↑ Gingrich, H. L.; Baum, J. S. In Oxazoles, Chemistry of Heterocyclic Compounds; Turchi, I. J., Ed.; Wiley: New York, 1986; Vol. 45. (doi:10.1002/9780470187289.ch4)
- ↑ Morin, M. S. T.; St-Cyr, D. J.; Arndtsen, B. A.; Krenske, E. H.; Houk, K. N., Modular Mesoionics: Understanding and Controlling Regioselectivity in 1,3-Dipolar Cycloadditions of Münchnone Derivatives. J. Am. Chem. Soc. 2013, 135, 17349. (doi:10.1021/ja406833q)