Sodium ascorbate
 | |
 | |
| Names | |
|---|---|
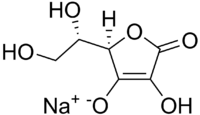
| IUPAC name
Sodium (2R)-2-[(1S)-1,2-dihydroxyethyl]-4-hydroxy-5-oxo-2H-furan-3-olate | |
| Other names
Sodascorbate; Monosodium ascorbate; E301 | |
| Identifiers | |
| 134-03-2 | |
| ChEMBL | ChEMBL591665 |
| EC Number | 205-126-1 |
| Jmol interactive 3D | Image |
| PubChem | 23667548 |
| RTECS number | CI7671000 |
| UNII | S033EH8359 |
| |
| |
| Properties | |
| C6H7NaO6 | |
| Molar mass | 198.11 g·mol−1 |
| Appearance | minute white to yellow crystals |
| Odor | odorless |
| Melting point | 218 °C (424 °F; 491 K) (decomposes) |
| 62 g/100 mL (25 °C) 78 g/100 mL (75 °C) | |
| Solubility | very slightly soluble in alcohol insoluble in chloroform, ether |
| Hazards | |
| NFPA 704 | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Sodium ascorbate is one of a number of mineral salts of ascorbic acid (vitamin C). The molecular formula of this chemical compound is C6H7NaO6. As the sodium salt of ascorbic acid, it is known as a mineral ascorbate. It has not been demonstrated to be more bioavailable than any other form of vitamin C supplement.[2]
Sodium ascorbate normally provides 131 mg of sodium per 1,000 mg of ascorbic acid (1,000 mg of sodium ascorbate contains 889 mg of ascorbic acid and 111 mg of sodium).
As a food additive, it has the E number E301 and is used as an antioxidant and an acidity regulator. It is approved for use as a food additive in the EU,[3] USA,[4] and Australia and New Zealand.[5]
In in vitro studies, sodium ascorbate has been found to produce cytotoxic effects in various malignant cell lines, which include melanoma cells that are particularly susceptible.[6][7]
Production
Sodium ascorbate is produced by dissolving ascorbic acid in water and adding an equivalent amount of sodium bicarbonate in water. After cessation of effervescence, the sodium ascorbate is precipitated by the addition of isopropanol.
References
- ↑ (+)-Sodium L-ascorbate at Sigma-Aldrich
- ↑ Linus Pauling Institute, Oregon Stare University: "Bioavailability of Different Forms of Vitamin C". Retrieved 2013-09-27.
- ↑ UK Food Standards Agency: "Current EU approved additives and their E Numbers". Retrieved 2011-10-27.
- ↑ US Food and Drug Administration: "Listing of Food Additives Status Part II". Retrieved 2011-10-27.
- ↑ Australia New Zealand Food Standards Code"Standard 1.2.4 - Labelling of ingredients". Retrieved 2011-10-27.
- ↑ Chen Q, Espey MG, Krishna MC, Mitchell JB, Corpe CP, Buettner GR, Shacter E, Levine M. (September 2005). "Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues". Proc Natl Acad Sci U S A. 102 (38): 13604–9. doi:10.1073/pnas.0506390102. PMC 1224653. PMID 16157892.
- ↑ Kang JS, Cho D, Kim YI, Hahm E, Kim YS, Jin SN, Kim HN, Kim D, Hur D, Park H, Hwang YI, Lee WJ. . (July 2005). "Sodium ascorbate (vitamin C) induces apoptosis in melanoma cells via the down-regulation of transferrin receptor dependent iron uptake". J Cell Physiol. 204 (1): 192–7. doi:10.1002/jcp.20286. PMID 15672419.
