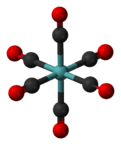
Molybdenum hexacarbonyl
| |||
| | |||
| Names | |||
|---|---|---|---|
| IUPAC name
Hexacarbonylmolybdenum(0) | |||
| Systematic IUPAC name
Hexacarbonylmolybdenum[1] | |||
| Identifiers | |||
| 13939-06-5 | |||
| ChEBI | CHEBI:30508 | ||
| ChemSpider | 21428397 | ||
| EC Number | 237-713-3 | ||
| 3798, 562210 | |||
| Jmol interactive 3D | Image | ||
| MeSH | Hexacarbonylmolybdenum | ||
| PubChem | 98885 | ||
| UN number | 3466 | ||
| |||
| |||
| Properties | |||
| C6MoO6 | |||
| Molar mass | 264.01 g·mol−1 | ||
| Appearance | Vivid, white, translucent crystals | ||
| Density | 1.96 g cm−3 | ||
| Melting point | 150 °C (302 °F; 423 K) | ||
| Boiling point | 156 °C (313 °F; 429 K) | ||
| Structure | |||
| Orthogonal | |||
| Octahedral | |||
| 0 D | |||
| Thermochemistry | |||
| Std enthalpy of formation (ΔfH |
-989.1 kJ mol−1 | ||
| Std enthalpy of combustion (ΔcH |
-2123.4 kJ mol−1 | ||
| Hazards | |||
| Safety data sheet | External MSDS | ||
| EU classification (DSD) |
| ||
| R-phrases | R26/27/28 | ||
| S-phrases | (S1/2), S36/37/39, S45 | ||
| Related compounds | |||
| Related compounds |
Chromium hexacarbonyl | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Molybdenum hexacarbonyl (also called molybdenum carbonyl) is the chemical compound with the formula Mo(CO)6. This colorless solid, like its chromium and tungsten analogues, is noteworthy as a volatile, air-stable derivative of a metal in its zero oxidation state.
Structure and properties
Mo(CO)6 adopts an octahedral geometry consisting of six rod-like CO ligands radiating from the central Mo atom. A recurring minor debate in some chemical circles concerns the definition of an "organometallic" compound. Usually, organometallic indicates the presence of a metal directly bonded via a M-C bond to an organic fragment, which must in turn have a C-H bond. By this strict definition, Mo(CO)6 is not organometallic.
Preparation
Mo(CO)6 is prepared by the reduction of molybdenum chlorides or oxides under a pressure of carbon monoxide, although it would be unusual to prepare this inexpensive compound in the laboratory. The compound is somewhat air-stable and sparingly soluble in nonpolar organic solvents.
Occurrence
Mo(CO)6 has been detected in landfills and sewage plants, the reducing, anaerobic environment being conducive to formation of Mo(CO)6.[2]
Applications in inorganic and organometallic synthesis
Molybdenum hexacarbonyl is widely used in electron beam-induced deposition technique - it is easily vaporized and decomposed by the electron beam providing a convenient source of molybdenum atoms.[3] Mo(CO)6 is also a popular reagent in organometallic synthesis[4] because one or more CO ligands can be displaced by other donor ligands.[5] For example, Mo(CO)6 reacts with 2,2'-bipyridine to afford Mo(CO)4(bipy). UV-photolysis of a THF solution of Mo(CO)6 gives Mo(CO)5(THF). Many metal carbonyls are similarly photo-activatable.
[Mo(CO)4(piperidine)2]
The thermal reaction of Mo(CO)6 with piperidine affords Mo(CO)4(piperidine)2. The two piperidine ligands in this yellow-colored compound are labile, which allows other ligands to be introduced under mild conditions. For instance, the reaction of [Mo(CO)4(piperidine)2] with triphenyl phosphine in boiling dichloromethane (b.p. ca. 40 °C) gives cis-[Mo(CO)4(PPh3)2]. This cis- complex isomerizes in toluene to trans-[Mo(CO)4(PPh3)2].[6]
[Mo(CO)3(MeCN)3]
Upon refluxing in a solution of acetonitrile, Mo(CO)6 converts to its tris(acetonitrile) derivative. The resulting air-sensitive compound serves as a source of "Mo(CO)3". For instance treatment with allyl chloride gives [MoCl(allyl)(CO)2(MeCN)2], whereas treatment with KTp and sodium cyclopentadienide gives [MoTp(CO)3]− and [MoCp(CO)3]− anions respectively. These anions can be reacted with electrophiles to form a wide range of products.[7]
Applications in organic synthesis
Mo(CO)6, [Mo(CO)3(MeCN)3], and related derivatives are employed as catalysts in organic synthesis. For example, these catalysts can be used for alkyne metathesis and the Pauson–Khand reaction.
Safety and handling
Like all metal carbonyls, Mo(CO)6 is dangerous source of volatile metal as well as CO. It diffuses readily into plastic stoppers.
References
- ↑ "Hexacarbonylmolybdenum (CHEBI:30508)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute.
- ↑ Feldmann, J. (1999). "Determination of Ni(CO)4, Fe(CO)5, Mo(CO)6, and W(CO)6 in Sewage Gas by Using Cryotrapping Gas Chromatography Inductively Coupled Plasma Mass Spectrometry". Journal of Environmental Monitoring 1 (1): 33–37. doi:10.1039/a807277i.
- ↑ Randolph, S. J.; Fowlkes, J. D.; Rack, P. D. (2006). "Focused, Nanoscale Electron-Beam-Induced Deposition and Etching". Critical Reviews of Solid State and Materials Sciences 31 (3): 55–89. doi:10.1080/10408430600930438.
- ↑ Faller, J. W.; Brummond, K. M.; Mitasev, B. (2006). "Hexacarbonylmolybdenum". In L. Paquette. Encyclopedia of Reagents for Organic Synthesis. New York: J. Wiley & Sons. doi:10.1002/047084289X.rh004.pub2.
- ↑ http://www.chm.bris.ac.uk/teaching-labs/inorganic2ndyear/2004-2005labmanual/Experiment3.pdf Archived March 9, 2008 at the Wayback Machine
- ↑ Darensbourg, D. J.; Kump, R. L. (1978). "A Convenient Synthesis of cis-Mo(CO)4L2 Derivatives (L = Group 5a Ligand) and a Qualitative Study of Their Thermal Reactivity toward Ligand Dissociation". Inorganic Chemistry 17 (9): 2680–2682. doi:10.1021/ic50187a062.
- ↑ Elschenbroich, C.; Salzer, A. (1992). Organometallics : A Concise Introduction (2nd ed.). Weinheim: Wiley-VCH. ISBN 3-527-28165-7.
Further reading
- Marradi, M. (2005). "SYNLETT Spotlight 119 Molybdenum Hexacarbonyl [Mo(CO)6]" (pdf). SYNLETT 2005 (7): 1195–1196. doi:10.1055/s-2005-865206.
- Feldmann, J.; Cullen, W. R. (1997). "Occurrence of Volatile Transition Metal Compounds in Landfill Gas: Synthesis of Molybdenum and Tungsten Carbonyls in the Environment". Environmental Science and Technology 31 (7): 2125–2129. doi:10.1021/es960952y.
- Feldmann, J.; Grümping, R.; Hirner, A. V. (1994). "Determination of Volatile Metal and Metalloid Compounds in Gases from Domestic Waste Deposits with GC/ICP-MS". Fresenius' Journal of Analytical Chemistry 350 (4–5): 228–234. doi:10.1007/BF00322474.
| ||||||||||||||||||||||||||