Molecular machine
| Part of a series of articles on |
| Molecular nanotechnology |
|---|
|
A molecular machine, or nanomachine,[1] is any discrete number of molecular components that produce quasi-mechanical movements (output) in response to specific stimuli (input).[2] The expression is often more generally applied to molecules that simply mimic functions that occur at the macroscopic level. The term is also common in nanotechnology where a number of highly complex molecular machines have been proposed that are aimed at the goal of constructing a molecular assembler. Molecular machines can be divided into two broad categories; synthetic and biological.
Molecular systems capable of shifting a chemical or mechanical process away from equilibrium represent a potentially important branch of chemistry and nanotechnology. As the gradient generated from this process is able to perform useful work these types of systems, by definition, are examples of molecular machinery.
Historical insight and studies
There are two thought experiments that form the historical basis for molecular machines: Maxwell's demon and Feynman's Ratchet (or Brownian ratchet). Maxwell's Demon is well described elsewhere, and a slightly different interpretation of Richard Feynman's ratchet is given here.
Imagine a very small system (seen below) of two paddles or gears connected by a rigid axle and that it is possible to keep these two paddles at two different temperatures. One of the gears (at T2) has a pawl that is rectifying the system motion, and therefore, the axle can only move in a clockwise rotation, and in doing so, it could lift a weight (m) upward upon ratcheting. Now imagine if the paddle in box T1 was in a much hotter environment than the gear in box T2; it would be expected that the kinetic energy of the gas molecules (red circles) hitting the paddle in T1 would be much higher than the gas molecules hitting the gear at T2. Therefore, with lower kinetic energy of the gases in T2, there would be very little resistance from the molecules on colliding with the gear in the statistically opposite direction. Further, the ratcheting would allow for directionality, and slowly over time, the axle would rotate and ratchet, lifting the weight (m).

As described, this system may seem like a perpetual motion machine; however, the key ingredient is the heat gradient within the system. This ratchet does not threaten the second law of thermodynamics, because this temperature gradient must be maintained by some external means. Brownian motion of the gas particles provides the power to the machine, and the temperature gradient allows the machine to drive the system cyclically away from equilibrium. In Feynman's ratchet, random Brownian motion is not fought against, but instead, harnessed and rectified. Unfortunately, temperature gradients cannot be maintained over molecular scale distances because of molecular vibration redistributing the energy to other parts of the molecule. Furthermore, despite Feynman's machine doing useful work in lifting the mass, using Brownian motion to power a molecular level machine does not provide any insight on how that power (or potential energy of the lifted weight, m) can be used to perform nanoscale tasks.
Modern insights and studies
Unlike macroscopic motion, molecular systems are constantly undergoing significant dynamic motions subject to the laws of Brownian mechanics (or Brownian motion), and as such, harnessing molecular motion is a far more difficult process. At the macroscopic level, many machines operate in the gas phase, and often, air resistance is neglected, as it is insignificant, but analogously for a molecular system in a Brownian environment, molecular motion is similar "to walking in a hurricane, or swimming in molasses." The phenomenon of Brownian motion (observed by Robert Brown, 1827) was later explained by Albert Einstein in 1905. Einstein found that Brownian motion is a consequence of scale and not the nature of the surroundings. As long as thermal energy is applied to a molecule, it will undergo Brownian motion with the kinetic energy appropriate to that temperature. Therefore, like Feynman's strategy, when designing a molecular machine, it seems sensible to utilize Brownian motion rather than attempt to fight against it.
Like macroscopic machines, molecular machines typically have movable parts. However, while everyday macroscopic machines may provide inspiration for molecular machines, it is misleading to draw analogies between their design strategy; the dynamics of large and small length scales are simply too different. Harnessing Brownian motion and making molecular level machines is regulated by the second law of thermodynamics, with its often counter-intuitive consequences, and as such, we need another inspiration.
Although it is a challenging process to harness Brownian motion, nature has provided us with several blueprints for molecular motion performing useful work. Nature has created many useful structures for compartmentalizing molecular systems, hence creating distinct non-equilibrium distributions; the cell membrane is an excellent example. Lipophilic barriers make use of a number of different mechanisms to power motion from one compartment to another.
Examples of molecular machines
From a synthetic perspective, there are two important types of molecular machines: molecular switches (or shuttles) and molecular motors. The major difference between the two systems is that a switch influences a system as a function of state, whereas a motor influences a system as function of trajectory. A switch (or shuttle) may appear to undergo translational motion, but returning a switch to its original position undoes any mechanical effect and liberates energy to the system. Furthermore, switches cannot use chemical energy to repetitively and progressively drive a system away from equilibrium where a motor can.
Synthetic
A wide variety of rather simple molecular machines have been synthesized by chemists. They can consist of a single molecule; however, they are often constructed for mechanically-interlocked molecular architectures, such as rotaxanes and catenanes. Carbon nanotube nanomotors have also been produced.[3]
- Molecular motors are molecules that are capable of unidirectional rotation motion powered by external energy input. A number of molecular machines have been synthesized powered by light or reaction with other molecules.
- A molecular propeller is a molecule that can propel fluids when rotated, due to its special shape that is designed in analogy to macroscopic propellers. It has several molecular-scale blades attached at a certain pitch angle around the circumference of a nanoscale shaft. Also see molecular gyroscope.
- A molecular switch is a molecule that can be reversibly shifted between two or more stable states. The molecules may be shifted between the states in response to changes in e.g. pH, light, temperature, an electric current, microenvironment, or the presence of a ligand.
- A molecular shuttle is a molecule capable of shuttling molecules or ions from one location to another. A common molecular shuttle consists of a rotaxane where the macrocycle can move between two sites or stations along the dumbbell backbone.
- Molecular tweezers are host molecules capable of holding items between its two arms. The open cavity of the molecular tweezers binds items using non-covalent bonding including hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, π-π interactions, and/or electrostatic effects. Examples of molecular tweezers have been reported that are constructed from DNA and are considered DNA machines.
- A molecular sensor is a molecule that interacts with an analyte to produce a detectable change.[4] Molecular sensors combine molecular recognition with some form of reporter, so the presence of the item can be observed.
- A molecular logic gate is a molecule that performs a logical operation on one or more logic inputs and produces a single logic output. Unlike a molecular sensor, the molecular logic gate will only output when a particular combination of inputs are present.
Biological
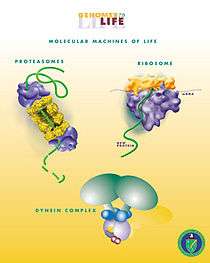
The most complex molecular machines are proteins found within cells. These include motor proteins, such as myosin, which is responsible for muscle contraction, kinesin, which moves cargo inside cells away from the nucleus along microtubules, and dynein, which produces the axonemal beating of motile cilia and flagella. These proteins and their nanoscale dynamics are far more complex than any molecular machines that have yet been artificially constructed.
Probably the most significant biological machine known is the ribosome. Other important examples include ciliary mobility. A high-level-abstraction summary is that, "[i]n effect, the [motile cilium] is a nanomachine composed of perhaps over 600 proteins in molecular complexes, many of which also function independently as nanomachines."[1] Flexible linker domains allow the connecting protein domains to recruit their binding partners and induce long-range allostery via protein domain dynamics. [5]
This protein flexibility allows the construction of biological machines. The first useful applications of these biological machines might be in nanomedicine. For example,[6] they could be used to identify and destroy cancer cells.[7][8] Molecular nanotechnology is a speculative subfield of nanotechnology regarding the possibility of engineering molecular assemblers, biological machines which could re-order matter at a molecular or atomic scale. Nanomedicine would make use of these nanorobots, introduced into the body, to repair or detect damages and infections. Molecular nanotechnology is highly theoretical, seeking to anticipate what inventions nanotechnology might yield and to propose an agenda for future inquiry. The proposed elements of molecular nanotechnology, such as molecular assemblers and nanorobots are far beyond current capabilities.[9][10]
Theoretical
The construction of more complex molecular machines is an active area of theoretical research. A number of molecules, such as molecular propellers, have been designed, although experimental studies of these molecules are inhibited by the lack of methods to construct these molecules. These complex molecular machines form the basis of areas of nanotechnology, including molecular assembler.
See also
References
- 1 2 Satir, Peter; Søren T. Christensen (2008-03-26). "Structure and function of mammalian cilia". Histochemistry and Cell Biology (Springer Berlin / Heidelberg) 129 (6): 688. doi:10.1007/s00418-008-0416-9. PMC 2386530. PMID 18365235. 1432-119X. Retrieved 2009-09-11.
- ↑ Ballardini R, Balzani V, Credi A, Gandolfi MT, Venturi M. (2001). "Artificial Molecular-Level Machines: Which Energy To Make Them Work?". Acc. Chem. Res. 34 (6): 445–455. doi:10.1021/ar000170g.
- ↑ Fennimore, A. M.; T.D. Yuzvinsky, Wei-Qiang Han, M. S. Fuhrer, J. Cumings and A. Zettl (2003). "Rotational actuators based on carbon nanotubes". Nature 424 (6947): 408–410. Bibcode:2003Natur.424..408F. doi:10.1038/nature01823. PMID 12879064. Cite uses deprecated parameter
|coauthors=(help) - ↑ Cavalcanti A, Shirinzadeh B, Freitas Jr RA, Hogg T. (2008). "Nanorobot architecture for medical target identification". Nanotechnology 19 (1): 015103(15pp). Bibcode:2008Nanot..19a5103C. doi:10.1088/0957-4484/19/01/015103.
- ↑ Bu Z, Callaway DJ (2011). "Proteins MOVE! Protein dynamics and long-range allostery in cell signaling". Adv in Protein Chemistry and Structural Biology. Advances in Protein Chemistry and Structural Biology 83: 163–221. doi:10.1016/B978-0-12-381262-9.00005-7. ISBN 9780123812629. PMID 21570668.
- ↑ Amrute-Nayak, M.; Diensthuber, R. P.; Steffen, W.; Kathmann, D.; Hartmann, F. K.; Fedorov, R.; Urbanke, C.; Manstein, D. J.; Brenner, B.; Tsiavaliaris, G. (2010). "Targeted Optimization of a Protein Nanomachine for Operation in Biohybrid Devices". Angewandte Chemie 122 (2): 322–326. doi:10.1002/ange.200905200.
- ↑ Patel, G. M.; Patel, G. C.; Patel, R. B.; Patel, J. K.; Patel, M. (2006). "Nanorobot: A versatile tool in nanomedicine". Journal of Drug Targeting 14 (2): 63–7. doi:10.1080/10611860600612862. PMID 16608733.
- ↑ Balasubramanian, S.; Kagan, D.; Jack Hu, C. M.; Campuzano, S.; Lobo-Castañon, M. J.; Lim, N.; Kang, D. Y.; Zimmerman, M.; Zhang, L.; Wang, J. (2011). "Micromachine-Enabled Capture and Isolation of Cancer Cells in Complex Media". Angewandte Chemie International Edition 50 (18): 4161–4164. doi:10.1002/anie.201100115.
- ↑ Freitas, Robert A., Jr.; Havukkala, Ilkka (2005). "Current Status of Nanomedicine and Medical Nanorobotics" (PDF). Journal of Computational and Theoretical Nanoscience 2 (4): 1–25. doi:10.1166/jctn.2005.001.
- ↑ Nanofactory Collaboration