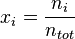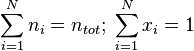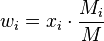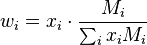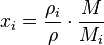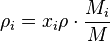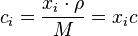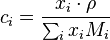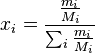Mole fraction
In chemistry, the mole fraction or molar fraction ( ) is defined as the amount of a constituent (expressed in moles),
) is defined as the amount of a constituent (expressed in moles), 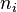 , divided by the total amount of all constituents in a mixture,
, divided by the total amount of all constituents in a mixture, 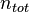 :[1]
:[1]
The sum of all the mole fractions is equal to 1:
The same concept expressed with a denominator of 100 is the mole percent or molar percentage or molar proportion (mol%).
The mole fraction is also called the amount fraction.[1] It is identical to the number fraction, which is defined as the number of molecules of a constituent  divided by the total number of all molecules
divided by the total number of all molecules 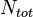 . The mole fraction is sometimes denoted by the lowercase Greek letter
. The mole fraction is sometimes denoted by the lowercase Greek letter  (chi) instead of a Roman
(chi) instead of a Roman  .[2][3] For mixtures of gases, IUPAC recommends the letter
.[2][3] For mixtures of gases, IUPAC recommends the letter 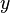 .[1]
.[1]
The National Institute of Standards and Technology of the United States prefers the term amount-of-substance fraction over mole fraction because it does not contain the name of the unit mole.[4]
Whereas mole fraction is a ratio of moles to moles, molar concentration is a ratio of moles to volume.
The mole fraction is one way of expressing the composition of a mixture with a dimensionless quantity; mass fraction (percentage by weight, wt%) and volume fraction (percentage by volume, vol%) are others.
Properties
Mole fraction is used very frequently in the construction of phase diagrams. It has a number of advantages:
- it is not temperature dependent (such as molar concentration) and does not require knowledge of the densities of the phase(s) involved
- a mixture of known mole fraction can be prepared by weighing off the appropriate masses of the constituents
- the measure is symmetric: in the mole fractions x=0.1 and x=0.9, the roles of 'solvent' and 'solute' are reversed.
- In a mixture of ideal gases, the mole fraction can be expressed as the ratio of partial pressure to total pressure of the mixture
Related quantities
Mass fraction
The mass fraction  can be calculated using the formula
can be calculated using the formula
where  is the molar mass of the component
is the molar mass of the component  and
and 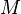 is the average molar mass of the mixture.
is the average molar mass of the mixture.
Replacing the expression of the molar mass:
Mole percentage
Multiplying mole fraction by 100 gives the mole percentage, also referred as amount/amount percent (abbreviated as n/n%).
Mass concentration
The conversion to and from mass concentration  is given by:
is given by:
where 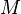 is the average molar mass of the mixture.
is the average molar mass of the mixture.
Molar concentration
The conversion to molar concentration 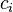 is given by:
is given by:
or
where 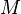 is the average molar mass of the solution, c total molar concentration and
is the average molar mass of the solution, c total molar concentration and  is the density of the solution .
is the density of the solution .
Mass and molar mass
The mole fraction can be calculated from the masses 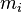 and molar masses
and molar masses  of the components:
of the components:
Spatial variation and gradient
In a spatially non-uniform mixture, the mole fraction gradient triggers the phenomenon of diffusion.
References
- 1 2 3 IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "amount fraction".
- ↑ Zumdahl, Steven S. (2008). Chemistry (8th ed.). Cengage Learning. p. 201. ISBN 0-547-12532-1.
- ↑ Rickard, James N. Spencer, George M. Bodner, Lyman H. (2010). Chemistry : structure and dynamics. (5th ed.). Hoboken, N.J.: Wiley. p. 357. ISBN 978-0-470-58711-9.
- ↑ Thompson, A.; Taylor, B. N. "The NIST Guide for the use of the International System of Units". http://physics.nist.gov/Pubs/SP811/sec08.html. National Institute of Standards and Technology. Retrieved 5 July 2014. External link in
|website=(help)
| ||||||
| ||||||||||||||||||