Mohs surgery
| Mohs surgery | |
|---|---|
| Intervention | |
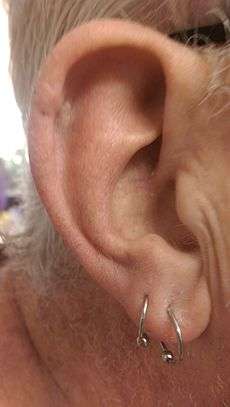 The ear of a 63 year old man, photo taken 16 days after Mohs surgery to remove a squamous cell carcinoma on the left upper edge of the ear, and three days after removal of the sutures | |
| MeSH | D015580 |
Mohs surgery, also known as chemosurgery, developed in 1938 by a general surgeon, Frederic E. Mohs, is microscopically controlled surgery used to treat common types of skin cancer. During the surgery, after each removal of tissue and while the patient waits, the tissue is examined for cancer cells. That examination informs the decision for additional tissue removal. Mohs surgery is one of the many methods of obtaining complete margin control during removal of a skin cancer (CCPDMA – complete circumferential peripheral and deep margin assessment.[1][2][3][4]) using frozen section histology.[5][6] CCPDMA or Mohs surgery allows for the removal of a skin cancer with very narrow surgical margin and a high cure rate.
The cure rate with Mohs surgery cited by most studies is between 97% and 99.8%[7] for primary basal cell carcinoma, the most common type of skin cancer. Mohs procedure is also used for squamous cell carcinoma, but with a lower cure rate. Two isolated studies reported cure rate for primary basal cell carcinoma as low as 95% and 96%.[8] Recurrent basal cell cancer has a lower cure rate with Mohs surgery, more in the range of 94%.[9] It has been used in the removal of melanoma-in-situ (cure rate 77% to 98% depending on surgeon), and certain types of melanoma (cure rate 52%).[10][11] Another study of melanoma-in-situ revealed Mohs cure rate of 95% for frozen section Mohs, and 98 to 99% for fixed tissue Mohs method.[12][13]
Other indications for Mohs surgery include dermatofibrosarcoma protuberans, keratoacanthoma, spindle cell tumors, sebaceous carcinomas, microcystic adnexal carcinoma, merkel cell carcinoma, Paget's disease of the breast, atypical fibroxanthoma, and leiomyosarcoma.[14][15] Because the Mohs procedure is micrographically controlled, it provides precise removal of the cancerous tissue, while healthy tissue is spared. Mohs surgery is also more cost effective than other surgical methods, when considering the cost of surgical removal and separate histopathological analysis. However, Mohs surgery should be reserved for the treatment of skin cancers in anatomic areas where tissue preservation is of utmost importance (face, hands, feet, genitals).[15]



Indications
Mohs surgery should not be used on the trunk or extremities for uncomplicated, non-melanoma skin cancer of less than one centimeter in size.[15][16] On these parts of the body, the risks exceed the benefits of the procedure.[15][16] Due to high risk of recurrence among other reasons, Mohs surgery could be considered for skin cancers on hands, feet, ankles, shins, nipples, or genitals.[15][16]
Technique
The American Academy of Dermatology has developed its first appropriate use criteria (AUC) on Mohs micrographic surgery in collaboration with the following organizations: American College of Mohs Surgery; American Society for Mohs Surgery; and the American Society for Dermatologic Surgery Association. More than 75 physicians contributed to the development of the Mohs surgery AUC, which were published in the Journal of the American Academy of Dermatology and Dermatologic Surgery.[17]
The Mohs procedure is a pathology sectioning method that allows for the complete examination of the surgical margin. It is different from the standard bread loafing technique of sectioning,[18] where random samples of the surgical margin are examined.[19][20]
Mohs surgery is performed in four steps:
- Surgical removal of tissue (Surgical Oncology)
- Mapping the piece of tissue, freezing and cutting the tissue between 5 and 10 micrometers using a cryostat, and staining with hematoxylin and eosin (H&E) or other stains (Including Toluidine Blue)
- Interpretation of microscope slides (Pathology)
- Possible reconstruction of the surgical defect (Reconstructive Surgery)
The procedure is usually performed in a physician's office under local anesthetic. A small scalpel is utilized to cut around the visible tumor. A very small surgical margin is utilized, usually with 1 to 1.5 mm of "free margin" or uninvolved skin. The amount of free margin removed is much less than the usual 4 to 6 mm required for the standard excision of skin cancers.[21] After each surgical removal of tissue, the specimen is processed, cut on the cryostat and placed on slides, stained with H&E and then read by the Mohs surgeon/pathologist who examines the sections for cancerous cells. If cancer is found, its location is marked on the map (drawing of the tissue) and the surgeon removes the indicated cancerous tissue from the patient. This procedure is repeated until no further cancer is found. The vast majority of cases are then reconstructed by the Mohs surgeon.
The method is well described in current references.[22][23][24] The mapping combined with the "smashing the pie pan" method of processing is the essence of CCPDMA surgery. If one imagines an aluminum pie pan as the blood covered surgical margin, and the top of the pie is the crust covered surface of the skin – the goal is to flatten the aluminum pie pan into one flat sheet, mark it, stain it, and examine it under the microscope. Another author uses the example of peeling the skin off an orange.[25] Imagine an orange cut in half as the CCPDMA layer. The peel is the surgical margin. One can remove this peel and flatten it out on a glass slide to examine the roots of the invasive cancer. The mapping is simply how one stains and labels the sections for a microscopic examination. The sections can be processed in one piece[26] (using relaxing incisions at multiple points, or hemisectioned like a "Pac-Man" figure),[27] cut in halves, cut in quarters, or cut in multiple pieces. Single piece processing is acceptable for small cancers, and multiple piece sectioning facilitates processing and prevent artifacts. Single piece sectioning prevents errors introduced by soft, hard-to-handle tissue; or from accidental dropping or mislabeling of specimen. Multiple sectioning prevents compression artifacts, separation of tissue, and other logistical problems with handling large thin sheets of frozen skin.
Some physicians believe that frozen section histology is the same as Mohs micrographic surgery, but it is not.[28] Mohs surgery is performed using fresh tissue while frozen section histology may or may not be CCPDMA. Non-CCPDMA histology usually utilizes a random tissue sampling technique called "bread loafing". Bread loafing is a statistical sampling method which examines less than 5% of the total surgical margin (imagine pulling 5 slices of bread out of a whole loaf of sliced bread and examining only those 5 slices to visualize the whole loaf). In CCPDMA processing, the entire surgical margin is examined (imagine one who examined the entire outside crust of the same loaf of bread). In statistical terms, the more slices of bread one examines, the lower the "false negative" rate will become.[29] False negatives occur when a pathologist reads cancer excision as "free of residual carcinoma", even though cancer might be present in the wound and missed because of the random sampling.[30] In reality, most pathology labs examine only 3 to 8 sections of the "loaf" in their margin determination. The alternatives to Mohs surgery are CCPDMA based surgical excision and non-CCPDMA surgical excision. Mohs and CCPDMA pathologists have perfected methods of examining the entire surgical margin.
Mohs surgery often leaves an open wound, which most often is reconstructed (closed) by the Mohs surgeon.
The Mohs Surgery Team
The team consists of the Mohs surgeon, histotechnician, and assisting nurses. The Mohs surgeon identifies the cancer and its margin, often with the aid of dermatoscopy. He or she removes the cancer under local anesthetic and prepares it for histology processing. This is accomplished by cutting the specimen (if required), staining the specimen for orientation, and sending it to the lab.
The histotechnician prepares the tissue for Mohs processing by flattening the surgical margin on a flat surface first. Then the flat surgical surface is mounted on a cryostat to be sectioned and prepared for glass slides to be read by the pathologist.
In Mohs surgery, the surgeon acts as the pathologist. He or she examines the slide for residual tumors, and marks the location of the tumor on the pathology report.
The Mohs surgeon most often functions as the reconstructive surgeon.
Staining Convention
Each surgeon has his or her own convention. A typical convention is as follows[31]
Epidermis l l l l l l l l l l BLUE - - - - - - - - - RED --------------------------------- YELLOW O O O O O O O GREEN X X X X X X X Missing Epidermis V V V V V V V V V V
Differences between histology of transverse sections and vertical sections
Sometimes, for confirmation purposes, a second opinion will be asked of a pathologist to review pathology slides from Mohs cases. Traditional histology of skin tissue uses vertical sectioning – with the subcutaneous tissue at the bottom and the epidermis at the top. Mohs surgery uses tangential or horizontal sectioning, which can confuse the pathologists trained in the traditional method.
First, one has to determine the method of chromacoding or color-coding. The orientation of the Mohs map must be able to distinguish between medial, lateral, superior, and inferior.
Next, one has to determine if the surgeon followed the convention of mounting only 2 sections per case; as preferred by some authors;[31] or did he/she perform serial sectioning through the block as preferred by other authors.[32] If serial sectioning is performed, the distance between sections should be confirmed. Some surgeons utilize 100 micrometres between each section, and some utilize 200 micrometres between the first two sections, and 100 micrometres between subsequent sections (10 crank of tissue set at 6 to 10 micrometre is roughly equal to 100 micrometres if one allows for physical compression due to the blade).
Next, one determines if the entire epidermal border is present. Ideally 100% of the epithelial border should be present. Convention requires at least 95% of the epidermis to be present.[32] However, some surgeons will make an exception for some missing epithelium at the apices of an elliptical excision around the specimen. Ideally, oval sections should be performed. However, for practical purposes on some lesions, a surgeon might cut the Mohs section to approximate the final closure defect. The apexes are often 1 cm or more from the tumor, so clear margins at the apices can be ignored. This is not the ideal by convention, but is appropriate on a case by case basis.
Next, one determines if the surgical margin is clear. With serial sectioning, one has to recreate the surgical specimen in a 3-dimensional way. The first section that touches the blade begins the 3-dimensional reconstruction. By using the 3-D reconstruction of the specimen, one can say that all the epithelial margin is present as one progresses from deep to superficial. If only 2 sections are present, ideally, both the sections should be clear. If the deeper section is positive, one has to ascertain the distance between the sections. Convention often calls for a clear margin of at least 200 micrometres. For ambiguous structures that resemble both adnexal structure and carcinoma, following the serial sections will allow for one to identify the structure as benign or malignant. With the 2 slides method, this might be impossible to perform, as no 3-D reconstruction is possible with only 2 sections.
Carcinoma appearance under Mohs micrographic sectioning can be difficult. Tangential cut of squamous cell can mimic squamous cell carcinoma (but without the atypia). Sections through the buds of hair follicles can resemble isolated islands of basal cell cancers, often even with retraction artifact. Serial section analysis is best for Mohs surgery.
Mohs surgery and blood thinners
The trend in skin surgery over the last 10 years has been to continue anticoagulants while performing skin surgery. Most cutaneous bleeding can be controlled with electrocautery, especially bipolar forceps. The benefit gained by ease of hemostasis is weighed against the risk of stopping anticoagulants; and it is generally preferred to continue anticoagulants.[33]
Risks and complications
Cure rate
Few individuals argue about the cure rate for Mohs, especially pathologists familiar with the procedure.[28] However, in recent years, a few authors suggested that Mohs surgery is no better than standard excision.[8] Extensive studies performed by Mohs involving thousands of patients with both fixed tissue and fresh tissue cases have been reported in the literature.[34] Other surgeons repeated the studies with also thousands of cases, with nearly the same results.[35]
Clinical 5 year cure rates with Mohs surgery:
- 4085 cases of primary and recurrent cancer of face, scalp, and neck. Cure rate of 96.6%.[36]
- 1065 cases of squamous cell carcinoma of face, scalp, and neck – cure rate 94.8%[37]
- 2075 cases of basal cell cancer of the nose both primary and recurrent, cure rate 99.1%.[38]
- Cure rate for basal cell cancer of the ear, less than 1 cm, 124 cases, cure rate 100%.[39]
- Cure rate of basal cell cancer of the ear, 1 to 2 cm, 170 cases, 100%. One needs to keep in mind that the cases performed by Mohs were for large and extensive tumors, often treated numerous times before by other surgeons. Regardless, his cure rate for small primary tumors either were 100% or near 100% when separated out from larger or recurrent tumors.
Experienced Mohs surgeons have reported cure rates for melanoma-in-situ from 95% to 98% (depending on if it is small MIS, or lentigo maligna variant), much higher than previously reported by Mikhail of 77%.[12]
These are only a small number of cases reported by Mohs, and numerous other articles by other authors have shown tremendous cure rates for primary basal cell carcinoma. Studies by Smeet, et al. showing a Mohs cure rate of about 95%, and another study in Sweden showing Mohs cure rate of about 94%.[40]
Cure rate variation
Some of Mohs' data revealed a cure rate as low as 96%, but these were often very large tumors, previously treated by other modalities. Some authors claim that their 5 year cure rate for primary basal cell cancer exceeded 99% while other noted more conservative cure rate of 97%. The quoted cure rate for Mohs surgery on previously treated basal cell cancer is about 94%.[41] Reasons for variations in the cure rate include the following.
- Modern frozen section method. Frozen section histology does not give the added margin of safety by the cytotoxic Mohs paste,[42] originally used by Mohs. This paste might have destroyed any residual cancer cells not detected by the pathologist.
- Missing epidermal margins. Ideally, the Mohs section should include 100% of the epidermal margin, but greater than 95% is often accepted.[32] Unfortunately, vigorous scrubbing, poorly controlled initial curettage, poor tissue health, technician's error, and surgeon's error can introduce areas missing epithelial margin. Some surgeons consider 70% epithelial margin acceptable, while others suggest 100% margin. In the ideal situation, 100% of the epithelial margin should be available to be reviewed on serial sectioning of the Mohs specimen.
- Misreading of the pathology slide. It is difficult to differentiate between a small island of basal cell carcinoma and a hair follicle structure. Many Mohs surgeon limits their tissue processing to include only 2 sections of tissue.[31] This severely hampers their ability to determine if a structure is a hair follicle or a carcinoma. Two histologic sections can not fully distinguish these two nearly identical structures,[43] and can lead to either "false negative" or "false positive" errors by either calling a section clear of tumor, or calling a section positive for tumor, respectively. Serial sectioning of the tumor is preferred by other surgeons.[32][44] Surgeons who perform serial sectioning through the block of tissue (usually 100 micrometres apart) are assured of the contiguous nature of his tumor and the distance of the tumor from the surgical margin, and is familiarized with the nature of the tumor. Serial sectioning also makes it easier to work with three-dimensional tumor with margins that are difficult to compress.
- Compression artifact, freezing artifact, cautery artifact, tissue folds, crush artifact from forceps, relaxing incision artifact, cartilage dropping out, fat compression, poor staining, dropping of tumor, etc.[45] These can be introduced as the tumor is "flattened". Stain can run from the surgical edge, and stain the surgical margin - giving a false impression that the entire surgical margin is clear, when it is not. While some surgeon unfamiliar with the "whole piece" or "PacMan"[27] methods of processing might suggest that multiple piece sectioning is better than one. The more tissue sections are cut, the more artifacts in staining and tissue malformation will be introduced. It is imperative for the surgeon to be fully familiar with tissue handling and processing; and not simply relying on a trained technologist to perform his sectioning.
- Hard-to-see tumor in heavy inflammatory infiltrate.[46] This can occur with squamous cell carcinoma, especially when complicated with local infection, or intrinsic lymphoproliferative disorders (chronic lymphocytic leukemia). Because of abnormal peripheral blood profile, response to inflammatory skin conditions with patients with myelomonocytic leukemia can have appearance of atypical cells at sites of inflammation, confusing the Mohs surgeon.[46]
- Perineural spread, and benign changes simulating perineural spread. Tumor spreading along a nerve can be difficult to visualize, and sometime benign plasma cells can surround the nerve, simulating cancer.[47]
- Anatomical area that is difficult to cut and process.[48] Examples would be the ear, and other three-dimensional structures like eyelids. The ability to make a scallop shaped incision is increasingly difficult when the surgical surface is no longer a flat plane, but is a three-dimensional rigid structure.
- Recurrent skin cancer with multiple islands of recurrence. This can occur with either previous excision, or after electrodesiccation and curettage. As these residual skin cancer are often bound in scar tissue, and present in multiple location in the scar of the previous surgical defect - they are no longer contiguous in nature. Some surgeons advocate the removal of the complete scar in the treatment of "recurrent" skin cancers. Others advocate removing only the island of local recurrence, and leaving the previous surgical scar behind. The decision is often made depending on the location of the tumor, and the goal of the patient and physician.
- Unreported or underreported recurrence. Many patients do not return to the original surgeon to report a recurrence. The consulting surgeon on the repeat surgery may not inform the first surgeon of the recurrence. The time it takes for a recurrent tumor to be visible to the patient might be 5 or more years. Quoted "cure" rates must be looked upon with the understanding that a 5 year cure rate might not necessary be correct. As basal cell carcinoma is a very slowly progressing tumor, a 5 year no recurrence rate might not be adequate. Longer follow up might be needed to detect a slow growing tumor left in the surgical scar.
- Poor training of the surgeon/pathologist/histotechnologist. While Mohs surgery is essentially a technical method of tissue handling and processing, the skill and training of the surgeon can greatly affect the outcome. Success requires a foundation of good tissue handling and good surgical skill and hemostasis, based on the tissue processing and staining technique. A surgeon without a good histotechnologist does not have access to sufficiently high quality information about the cancer, and a histotechnologist without a good surgeon can not produce quality slides. Originally, surgeons learned the procedure by spending a few hours to several months with Mohs;.[49] Today, surgeons complete residency and fellowship spending hundreds of hours observing and performing Mohs surgery. It is highly encouraged that a physician interested in learning Mohs surgery should spend extended time observing, cutting, processing, and staining Mohs specimens. The histology block should be correctly mounted and cut the first time, as there is no second chance in Mohs histology. It is not a procedure that can be taught or learned in a short period of time. Many residency and Mohs fellowship continue to teach the processing of only 2 Mohs sections per tumor.[31]
Irrespective of the problems associated with Mohs surgery, a true cure rate approaching 100% can occur with primary basal cell carcinoma (previously untreated) if proper respect of the physician's limitation, the procedure's limitation, and his laboratory staff's limitation is paid. A conservative approach such as serial sectioning, good staining technique, and conservative Mohs margin (example: tumor at least 200 micrometre from the surgical margin) can assure the lowest recurrence rate.
Comparison to other modalities of treatment




Mohs surgery is not the answer for all skin cancers.[15] Under select circumstances, radiation, topical chemotherapy, cryosurgery, electrodesiccation and curettage, and standard excision are better than Mohs surgery. Studies comparing the effectiveness of Mohs surgery to other modalities often fail to specify surgical margin, method of processing (bread loafing with 3 or 4 sections, bread loafing with 0.1 mm spacing, margin controlled, frozen section vs. standard histology); leaving little argument one way or another. Once a pathologist understands the simplistic nature of Mohs surgery, and its margin control ability – little need is called for clinical trial comparing Mohs surgery to surgical excision.
In reality, Mohs micrographic surgery is nothing more than frozen section histology using a unique peripheral margin control tissue processing technique. There is nothing magical about its cure rate or why years of training is required. When compared to many other described peripheral margin control tissue processing technique – the end result is the same – allowing for the complete examination of 100% of the surgical margin. The method is unique only in that it is a simple way to handle soft, hard to cut tissue. Once learned, any pathologists currently doing frozen section histology would realize how simple the technique is. It is better than doing serial bread loafing at 0.1 mm interval for improved false negative error rate simply in requiring less time, less tissue handling, and fewer glass slides mounted. Once mounted as tangential or horizontal sections, the pathologist simply has to relearn how to visualize skin structure on a tangential to horizontal view. In the absence of a Mohs trained pathologist, peripheral sectioning followed by horizontal sectioning of the remaining center is equivalent to the Mohs method.[25][28][50]
The clinical quotes for cure rate of Mohs surgery is from 97% to 99.8% after 5 years for newly diagnosed basal cell cancer (BCC), decreasing to 94% or less for recurrent basal cell cancer. Radiation oncologists quote cure rate from 90 to 95% for BCCs less than 1 or 2 cm, and 85 to 90% for BCCs larger than 1 or 2 cm. Surgical excision cure rate varies from 99% for wide margin (4 to 6 mm) and small tumor, to as low as 70% for narrow margins applied to large tumors. Here the weakness of the procedure is the histopathological processing, and not the surgeon himself. The fault of the surgeon is lack of understanding pathology laboratory methods, and failing to follow the standard of care for adequate surgical margin. Usually, the cure rate using standard bread loafing is very low for narrow surgical margin and a large tumor, and very high for large margins on small tumors.[51][52][53] It is the pathology lab that makes the difference, especially when frozen section is utilized in the operating theater. A randomized study assigning patients with recurrent facial basal cell cancer to either Mohs surgery or standard excision revealed no statistical difference in the treatment of primary basal cell carcinoma. It found a higher cure rate with Mohs surgery in the treatment of recurrent basal cell carcinoma (5 year recurrence rate of 2.4% for Mohs vs 12.1% for standard).[54]
Cosmetic appearance for Mohs surgery is very good, if combined with good reconstructive surgical skills. The majority of facial reconstruction due to skin cancer is are performed by the Mohs surgeon. Other specialists that sometimes reconstruct skin cancer defects include oculoplastic surgeons, facial plastic surgeons and plastic surgeons. In certain area, the tip of the nose, and the nasal ala, Mohs surgery can result in significant deformity, and might require multiple staged reconstruction to rebuild the nose cosmetically.
In choosing a Mohs surgeon or reconstructive surgeon, it is mandatory that a patient request to see pictorial representation of his/her previous work. Then one can proceed to make a decision whether the Mohs surgeon should also be the reconstructive surgeon.
Society and culture
Overuse and costs
Some dermatologists, oncologists and health economists say that Mohs surgery is overused, because it is highly profitable. Dermatologists may perform the surgery on areas of the skin where it is not necessary, or perform unwanted cosmetic surgery. The incidence of Mohs surgery increased by more than 400% in 10 years. In a sample of 100 Mohs surgeries, the cost ranged from $7,594 to $474, with the higher costs for hospital-based physicians. In 2010, hospital-based dermatologists who did Mohs surgery were paid $586,083, according to Becker's Hospital Review. When the surgery is performed in different steps by different specialists, each specialist can bill for his or her own procedure, such as the surgery itself, closing the wound, anesthesiology, and facilities fees. This can total more than $25,000 for a single procedure.[55]
Associations
The American College of Mohs Surgery is an organization of dermatologists who perform dermatology and Mohs surgery in their practice. The organization sets standards of care for fellowship trained Mohs surgeons who perform Mohs surgery as a primary function of their practice. Completion of a current ACMS fellowship require 1 to 2 years of training in the Mohs procedure including reconstructive and plastic surgery – in addition to 4 years of residency.[56] Fellowship trained Mohs surgeons that belong to the ACMS are viewed by Primary Care Physicians as the best specialists to perform Mohs surgery.[57] ASMS Mohs surgeons are certified by a written and practical exam, and are required to submit to yearly peer review of their cases.[58]
The American Board of Medical Subspecialities is in the process of reviewing Mohs micrographic surgery as a separate subspecialty.
The American Academy of Dermatology is the largest organization of board certified dermatologists, with 17,140 members in the United States and internationally. Of these, 12% perform dermatologic and Mohs micrographic surgery.
The American Osteopathic College of Dermatology is the only organization that recognizes Mohs surgery as a separate subspecialty. The organization offers board certification exam through the auspice of the American Osteopathic Association.[59]
The American Society for Dermatologic Surgery founded in 1970 is the largest organization of board certified dermasurgeons with over 5000 members who perform dermatologic surgeries including Mohs micrographic surgery.
The Association of Academic Dermatologic Surgeons has board certified dermasurgeon professors who have faculty appointments at major teaching hospitals and universities and are engaged in training medical students and residents in the practice of dermatologic surgery and Mohs micrographic surgery.
History
Originally, Mohs used a chemical paste (an escharotic agent) to cauterize and kill the tissue. It was made of zinc chloride and bloodroot (the root of the plant Sanguinaria canadensis, which contains the alkaloid sanguinarine). The original ingredients were 40.0 gm Stibnite, 10.0 gm Sanguinaria canadensis, and 34.5 ml of saturated zinc chloride solution.[60]
This paste is similar to black salve or "Hoxsey's paste" (see Hoxsey Therapy), a fraudulent patent medicine, but its usage is different. Hoxsey used the paste for long periods, a harmful practice that was rapidly discredited.[61] Mohs left the paste on the wound only overnight, and the following day, the cancer and surrounding skin would be anesthetized and the cancer removed. The specimen was then excised, and the tissue examined under the microscope. If cancer remained, more paste was applied, and the patient would return the following day. Later, local anesthetic and frozen section histopathology applied to fresh tissue allowed the procedure to be performed the same day, with less tissue destruction, and similar cure rate.[19] The term "chemosurgery" remains today, and is used synonymously with Mohs micrographic surgery.
Research
Mohs surgery can be applied to any relatively non-aggressive locally invasive tumor with a contiguous growth pattern (i.e. no skipped growth, or metastasis). Today, most Mohs procedures are performed by dermatologists. However, pathologists, plastic surgeons, and otolaryngologists[62] have been trained and are utilizing Mohs surgery in their practice as well. Hopefully, as more physicians are trained in the method, Mohs surgery can be applied to other organs systems beside the skin. The limitation of this application to other tumors (i.e. prostate cancer, cervical cancer, laryngeal cancer) is that the tumor must be in the earliest stages and no metastasis has occurred. The second problem with Mohs surgery is the prolonged procedural time. However this is generally viewed as preferable to having a patient under general anesthesia which is more invasive for the patient and more costly to the healthcare system.
Currently, the American College of Mohs Surgery has limited training to physicians who have done a dermatology residency.[63] It is the only formal training program that ensures the person performing Mohs surgery has spent 1–2 years of dedicated study under a Mohs surgeon, although this still is no guarantee that this physician's outcomes are better than a Mohs surgeon who has chosen not to join the Mohs College. The American Society for Mohs Surgery continues to encourage the training of physicians of all specialties to learn and apply the method invented by Frederic Mohs. The argument against physicians of other specialties than dermatology gaining Mohs training is that they are not adequately trained in dermatopathology. The argument for training other physicians beside dermatologists is that most Mohs surgeons do not make the initial diagnosis of the skin cancer – thus misdiagnosis can be avoided. The pathology of Mohs sections are then limited to a few easily identifiable cancers, and the pathology of normal skin is simple enough to gain in a short preceptorship.
Mohs, a general surgeon, encouraged physicians of all surgical specialties to learn and apply his technique to the treatment of skin cancer. It is understood that he never intended to limit his method to be utilized by dermatologists alone. In countries where dermatology is not well developed, the Mohs procedure can easily be learned by any surgeon or pathologist after a short preceptorship – the same way Mohs taught many current Mohs surgeons.
Quotation from Mohs first book:
"The book should be useful to physicians who may be called on to treat or advise regarding treatment of skin cancers and other conditions that are described. This includes dermatologists, surgeons, plastic surgeons, otolaryngologists, gynecologists, urologists, proctologists, pathologists, internists, and general practitioners."[64]
References
- ↑ http://wwwu.tsgh.ndmctsgh.edu.tw/commcpc/images/nccn/Non-Melanoma%20Skin%20Cancer-2007.pdf[]
- ↑ http://wwwu.tsgh.ndmctsgh.edu.tw/commcpc/images/nccn/dfsp%20NCCN%202004.pdf[]
- ↑ Basal Cell and Squamous Cell Skin Cancers (PDF). National Comprehensive Cancer Network. November 12, 2006. pp. BCC–2, BCC–3. ISBN 0-683-08888-2.
- ↑ Dhingra N, Gajdasty A, Neal JW, Mukherjee AN, Lane CM (June 2007). "Confident complete excision of lid‐margin BCCs using a marginal strip: an alternative to Mohs' surgery". The British Journal of Ophthalmology 91 (6): 794–6. doi:10.1136/bjo.2006.109892. PMC 1955612. PMID 17229804.
- ↑ Bentkover SH, Grande DM, Soto H, Kozlicak BA, Guillaume D, Girouard S (2002). "Excision of head and neck basal cell carcinoma with a rapid, cross-sectional, frozen-section technique". Archives of Facial Plastic Surgery 4 (2): 114–9. doi:10.1001/archfaci.4.2.114. PMID 12020207.
- ↑ Minton TJ (August 2008). "Contemporary Mohs surgery applications". Current Opinion in Otolaryngology & Head and Neck Surgery 16 (4): 376–80. doi:10.1097/MOO.0b013e3283079cac. PMID 18626258.
- ↑ Mikhail, George R.; Mohs, Frederic Edward (1991). Mohs micrographic surgery. Philadelphia: W.B. Saunders. p. 13. ISBN 978-0-7216-3415-9.
- 1 2 Smeets NW, Krekels GA, Ostertag JU, et al. (2004). "Surgical excision vs Mohs' micrographic surgery for basal-cell carcinoma of the face: randomised controlled trial". Lancet 364 (9447): 1766–72. doi:10.1016/S0140-6736(04)17399-6. PMID 15541449.
- ↑ Mikhail, George R.; Mohs, Frederic Edward (1991). Mohs micrographic surgery. Philadelphia: W.B. Saunders. p. 7. ISBN 978-0-7216-3415-9.
- ↑ Mikhail, George R.; Mohs, Frederic Edward (1991). Mohs micrographic surgery. Philadelphia: W.B. Saunders. p. 4. ISBN 978-0-7216-3415-9.
- ↑ Gross, Kenneth Gary; Steinman, Howard K.; Rapini, Ronald P. (1999). Mohs Surgery: Fundamentals and Techniques. Saint Louis: Mosby. pp. 211–20. ISBN 978-0-323-00012-3.
- 1 2 Bene NI, Healy C, Coldiron BM (May 2008). "Mohs micrographic surgery is accurate 95.1% of the time for melanoma in situ: a prospective study of 167 cases". Dermatologic Surgery 34 (5): 660–4. doi:10.1111/j.1524-4725.2007.34124.x. PMID 18261099.
- ↑ Stevenson O, Ahmed I (2005). "Lentigo maligna : prognosis and treatment options". American Journal of Clinical Dermatology 6 (3): 151–64. doi:10.2165/00128071-200506030-00002. PMID 15943492.
- ↑ Gross, Kenneth Gary; Steinman, Howard K.; Rapini, Ronald P. (1999). Mohs Surgery: Fundamentals and Techniques. Saint Louis: Mosby. pp. 193–203. ISBN 978-0-323-00012-3.
- 1 2 3 4 5 6 "Mohs surgery appropriate use criteria". aad.org.
- 1 2 3 American Academy of Dermatology (February 2013), "Five Things Physicians and Patients Should Question", Choosing Wisely: an initiative of the ABIM Foundation (American Academy of Dermatology), retrieved 5 December 2013, which cites
- Connolly SM, Baker DR, Coldiron BM, et al. (October 2012). "AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery". Journal of the American Academy of Dermatology 67 (4): 531–50. doi:10.1016/j.jaad.2012.06.009. PMID 22959232.
- National Comprehensive Cancer Network (2011), National Comprehensive Cancer Network clinical practice guidelines in oncology (NCCN Guidelines): Basal cell and squamous cell skin cancers (PDF), Fort Washington, Pennsylvania: National Comprehensive Cancer Network, retrieved 5 December 2013

- ↑ "Appropriate use criteria". aad.org.
- ↑ Maloney, Mary E. (1999). Surgical dermatopathology. Malden, Mass: Blackwell Science. pp. 112–3. ISBN 978-0-86542-299-5.
- 1 2 Mikhail, George R.; Mohs, Frederic Edward (1991). Mohs micrographic surgery. Philadelphia: W.B. Saunders. pp. 3–4. ISBN 978-0-7216-3415-9.
- ↑ Bowen GM, White GL, Gerwels JW (September 2005). "Mohs micrographic surgery". American Family Physician 72 (5): 845–8. PMID 16156344.
- ↑ O'Reilly, Susan E.; Knowling, Meg (March 4, 2005). "Basal Cell Carcinoma". Cancer Management Guidelines. BC Cancer Agency.
- ↑ Maloney, Mary E. (1999). Surgical dermatopathology. Malden, Mass: Blackwell Science. pp. 1111–7. ISBN 978-0-86542-299-5.
- ↑ Gross, Kenneth Gary; Steinman, Howard K.; Rapini, Ronald P. (1999). Mohs Surgery: Fundamentals and Techniques. Saint Louis: Mosby. pp. 49–66. ISBN 978-0-323-00012-3.
- ↑ Mikhail, George R.; Mohs, Frederic Edward (1991). Mohs micrographic surgery. Philadelphia: W.B. Saunders. pp. 13–5. ISBN 978-0-7216-3415-9.
- 1 2 Maloney, Mary E. (1999). Surgical dermatopathology. Malden, Mass: Blackwell Science. p. 113. ISBN 978-0-86542-299-5.
- ↑ Gross, Kenneth Gary; Steinman, Howard K.; Rapini, Ronald P. (1999). Mohs Surgery: Fundamentals and Techniques. Saint Louis: Mosby. pp. 83–4. ISBN 978-0-323-00012-3.
- 1 2 Gross, Kenneth Gary; Steinman, Howard K.; Rapini, Ronald P. (1999). Mohs Surgery: Fundamentals and Techniques. Saint Louis: Mosby. pp. 86–9. ISBN 978-0-323-00012-3.
- 1 2 3 Maloney, Mary E. (1999). Surgical dermatopathology. Malden, Mass: Blackwell Science. p. 116. ISBN 978-0-86542-299-5.
- ↑ Maloney, Mary E. (1999). Surgical dermatopathology. Malden, Mass: Blackwell Science. p. 114. ISBN 978-0-86542-299-5.
- ↑ Maloney, Mary E. (1999). Surgical dermatopathology. Malden, Mass: Blackwell Science. p. 118. ISBN 978-0-86542-299-5.
- 1 2 3 4 Mikhail, George R.; Mohs, Frederic Edward (1991). Mohs micrographic surgery. Philadelphia: W.B. Saunders. p. 307. ISBN 978-0-7216-3415-9.
- 1 2 3 4 Gross, Kenneth Gary; Steinman, Howard K.; Rapini, Ronald P. (1999). Mohs Surgery: Fundamentals and Techniques. Saint Louis: Mosby. p. 62. ISBN 978-0-323-00012-3.
- ↑ Alcalay J (August 2001). "Cutaneous surgery in patients receiving warfarin therapy". Dermatologic Surgery 27 (8): 756–8. doi:10.1046/j.1524-4725.2001.01056.x. PMID 11493301.
- ↑ Mohs, Frederic Edward (1978). Chemosurgery: microscopically controlled surgery for skin cancer. Springfield, Ill: Thomas. ISBN 978-0-398-03725-3.
- ↑ Mikhail, George R.; Mohs, Frederic Edward (1991). Mohs micrographic surgery. Philadelphia: W.B. Saunders. ISBN 978-0-7216-3415-9.
- ↑ Mohs, Frederic Edward (1978). Chemosurgery: microscopically controlled surgery for skin cancer. Springfield, Ill: Thomas. p. 55. ISBN 978-0-398-03725-3.
- ↑ Mohs, Frederic Edward (1978). Chemosurgery: microscopically controlled surgery for skin cancer. Springfield, Ill: Thomas. p. 57. ISBN 978-0-398-03725-3.
- ↑ Mohs, Frederic Edward (1978). Chemosurgery: microscopically controlled surgery for skin cancer. Springfield, Ill: Thomas. p. 79. ISBN 978-0-398-03725-3.
- ↑ Mohs, Frederic Edward (1978). Chemosurgery: microscopically controlled surgery for skin cancer. Springfield, Ill: Thomas. p. 101. ISBN 978-0-398-03725-3.
- ↑ Wennberg AM, Larkö O, Stenquist B (September 1999). "Five-year results of Mohs' micrographic surgery for aggressive facial basal cell carcinoma in Sweden". Acta Dermato-venereologica 79 (5): 370–2. doi:10.1080/000155599750010292. PMID 10494714.
- ↑ Mikhail, George R.; Mohs, Frederic Edward (1991). Mohs micrographic surgery. Philadelphia: W.B. Saunders. pp. 6–7. ISBN 978-0-7216-3415-9.
- ↑ McDaniel S, Goldman GD (December 2002). "Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer". Archives of Dermatology 138 (12): 1593–6. doi:10.1001/archderm.138.12.1593. PMID 12472348.
- ↑ Maloney, Mary E. (1999). Surgical dermatopathology. Malden, Mass: Blackwell Science. pp. 134, 453. ISBN 978-0-86542-299-5.
- ↑ Gross, Kenneth Gary; Steinman, Howard K.; Rapini, Ronald P. (1999). Mohs Surgery: Fundamentals and Techniques. Saint Louis: Mosby. p. 272. ISBN 978-0-323-00012-3.
- ↑ Maloney, Mary E. (1999). Surgical dermatopathology. Malden, Mass: Blackwell Science. pp. 149–62. ISBN 978-0-86542-299-5.
- 1 2 Maloney, Mary E. (1999). Surgical dermatopathology. Malden, Mass: Blackwell Science. p. 446. ISBN 978-0-86542-299-5.
- ↑ Maloney, Mary E. (1999). Surgical dermatopathology. Malden, Mass: Blackwell Science. pp. 446–7. ISBN 978-0-86542-299-5.
- ↑ Mohs, Frederic Edward (1956). Chemosurgery in cancer, gangrene and infections: featuring a new method for the microscopically controlled excision of cancer. Springfield, Ill: Thomas. p. 16. OCLC 488726321.
- ↑ Gross, Kenneth Gary; Steinman, Howard K.; Rapini, Ronald P. (1999). Mohs Surgery: Fundamentals and Techniques. Saint Louis: Mosby. p. 4. ISBN 978-0-323-00012-3.
- ↑ Gross DA, Field LM (October 1987). "Cooperative frozen section surgery". The Journal of Dermatologic Surgery and Oncology 13 (10): 1085–8. doi:10.1111/j.1524-4725.1987.tb00915.x. PMID 3655078.
- ↑ Maloney, Mary E. (1999). Surgical dermatopathology. Malden, Mass: Blackwell Science. p. 110. ISBN 978-0-86542-299-5.
- ↑ Kimyai-Asadi A, Katz T, Goldberg LH, et al. (December 2007). "Margin involvement after the excision of melanoma in situ: the need for complete en face examination of the surgical margins". Dermatologic Surgery 33 (12): 1434–9; discussion 1439–41. doi:10.1111/j.1524-4725.2007.33313.x. PMID 18076608.
- ↑ Kimyai-Asadi A, Goldberg LH, Jih MH (September 2005). "Accuracy of serial transverse cross-sections in detecting residual basal cell carcinoma at the surgical margins of an elliptical excision specimen". Journal of the American Academy of Dermatology 53 (3): 469–74. doi:10.1016/j.jaad.2005.02.049. PMID 16112355.
- ↑ Mosterd K, Krekels GA, Nieman FH, et al. (December 2008). "Surgical excision versus Mohs' micrographic surgery for primary and recurrent basal-cell carcinoma of the face: a prospective randomised controlled trial with 5-years' follow-up". The Lancet Oncology 9 (12): 1149–56. doi:10.1016/S1470-2045(08)70260-2. PMID 19010733.
- ↑ Paying Till It Hurts. The High Earners. Part 6: Dermatology. Patients’ Costs Skyrocket; Specialists’ Incomes Soar. By Elisabeth Rosenthal, New York Times, Jan. 18, 2014
- ↑ "The Mohs College Difference". Skincancermohssurgery.org. Retrieved 2012-09-19.
- ↑ Ibrahimi OA, Bangash H, Green L, Alam M, Armstrong AW, Eisen DB (October 2012). "Perceptions of expertise in cutaneous surgery and cosmetic procedures: what primary care physicians think". Dermatologic Surgery 38 (10): 1645–51. doi:10.1111/j.1524-4725.2012.02577.x. PMID 22958115.
- ↑ "Peer Review Program - American Society for Mohs Surgery". Mohssurgery.org. Retrieved 2012-09-19.
- ↑ "Dermatology: Definition of specialty practice". American Osteopathic Information Association and American Osteopathic Association.
MOHS micrographic surgery is a subspecialty of dermatology that utilizes a modified type of surgical excision combined with a modified frozen section technique which offers the highest available cure rates for both primary and recurrent skin cancers
- ↑ Mohs, Frederic Edward (1978). Chemosurgery: microscopically controlled surgery for skin cancer. Springfield, Ill: Thomas. pp. 3–6. ISBN 978-0-398-03725-3.
- ↑ "This Week in FDA History". U.S. Food and Drug Administration. Archived from the original on 8 November 2006. Retrieved 2008-08-27.
- ↑ Gross, Kenneth Gary; Steinman, Howard K.; Rapini, Ronald P. (1999). Mohs Surgery: Fundamentals and Techniques. Saint Louis: Mosby. pp. 248–60. ISBN 978-0-323-00012-3.
- ↑ "Fellowship Training". American College of Mohs Surgery.
- ↑ Mohs, Frederic Edward (1956). Chemosurgery in cancer, gangrene and infections: featuring a new method for the microscopically controlled excision of cancer. Springfield, Ill: Thomas. p. vii. OCLC 488726321.
External links
- American Cancer Society statement about treating basal cell carcinoma, including via Mohs surgery
- American Society for Mohs Surgery
- American College of Mohs Surgery
| ||||||||||||||||||||