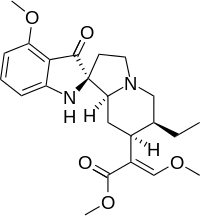
Mitragynine pseudoindoxyl
 | |
| Names | |
|---|---|
| IUPAC name
Methyl (E)-2-((1′S,6′S,7′S,8a′S)-6'-ethyl-4-methoxy-3-oxo-3',5',6',7',8',8a'-hexahydro-2′H-spiro[indoline-2,1'-indolizin]-7'-yl)-3-methoxyacrylate | |
| Other names
spiro[2H-indole-2,1'(5′H)-indolizine]-7'-acetic acid, 6'-ethyl-1,2',3,3',6',7',8',8'a-octahydro-4-methoxy-α-(methoxymethylene)-3-oxo-, methyl ester, (αE,2S,6′S,7′S,8′aS) | |
| Identifiers | |
| ChEMBL | ChEMBL58362 |
| ChemSpider | 23152339 |
| Jmol interactive 3D | Image |
| PubChem | 44301701 |
| |
| |
| Properties | |
| C23H30N2O5 | |
| Molar mass | 414.50 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Mitragynine pseudoindoxyl is a semi-synthetic opioid compound derived from natural mitragynine found in the plant Mitragyna speciosa, commonly known as Kratom.[1]
Pharmacology
Mitragynine pseudoindoxyl itself acts primarily via mu and delta opioid receptors, being more potent than morphine and about as potent as enkephalin.[2][3]
See also
References
- ↑ Jansen KL, Prast CJ (1988). "Ethnopharmacology of kratom and the Mitragyna alkaloids". J Ethnopharmacol 23 (1): 115–9. doi:10.1016/0378-8741(88)90121-3. PMID 3419199.
- ↑ Takayama H, Ishikawa H, Kurihara M, Kitajima M, Aimi N, Ponglux D, Koyama F, Matsumoto K, Moriyama T, Yamamoto LT, Watanabe K, Murayama T, Horie S (April 2002). "Studies on the synthesis and opioid agonistic activities of mitragynine-related indole alkaloids: discovery of opioid agonists structurally different from other opioid ligands". J. Med. Chem. 45 (9): 1949–56. doi:10.1021/jm010576e. PMID 11960505.
- ↑ Yamamoto, L. T.; Horie, S.; Takayama, H.; Aimi, N.; Sakai, S.; Yano, S.; Shan, J.; Pang, P. K.; Ponglux, D.; Watanabe, K. (1999). "Opioid receptor agonistic characteristics of mitragynine pseudoindoxyl in comparison with mitragynine derived from Thai medicinal plant Mitragyna speciosa". General pharmacology 33 (1): 73–81. doi:10.1016/S0306-3623(98)00265-1. PMID 10428019.
This article is issued from Wikipedia - version of the Monday, November 02, 2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.