Selenol

Selenols are organic compounds that contain the functional group with the connectivity C–Se–H. Selenols are sometimes also called selenomercaptans and selenothiols. Selenols are one of the principal classes of organoselenium compounds. The best known member is the amino acid selenocysteine.
Structure, bonding, properties
Selenols are structurally similar to thiols, but the C-Se bond is about 8% longer at 196 pm. The C–Se–H angle approaches 90° as it does in hydrogen selenide (H2Se). The bonding involves almost pure p-orbitals on Se, hence the near 90 angles. The Se–H bond energy is weaker than the S–H bond, consequently selenols are easily oxidized and serve as H-atom donors. Also reflecting the relative weakness of bonds to Se, selenols are about 1000x stronger acids than are thiols: the pKa of CH3SeH is 5.2 vs 8.3 for CH3SH. Deprotonation affords the selenolate anion, RSe−, most examples of which are highly nucleophilic and rapidly oxidized by air.[1]
The boiling points of selenols tend to be slightly greater than for thiols owing to the increased importance of van der Waals bonding, which is stronger for larger atoms. Volatile selenols have highly offensive odors.
Applications and occurrence
Selenols enjoy few commercial applications, being limited by the high toxicity of selenium as well as the sensitivity of the Se–H bond. Their conjugate bases, the selenolates, do enjoy limited applications in organic synthesis.
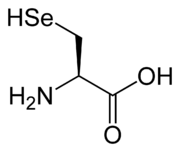
Biochemical role
Selenols are important in certain biological processes. Three enzymes found in mammals contain selenols at their active sites: glutathione peroxidase, iodothyronine deiodinase, and thioredoxin reductase. The selenols in these proteins are part of the essential amino acid selenocysteine.[1] The selenols function as reducing agents to give selenenic acid derivative (RSe–OH), which in turn are re-reduced by thiol-containing enzymes. Methaneselenol (commonly named "methylselenol") (CH3SeH), which can be produced in vitro by incubating selenomethionine with a bacterial methionine gamma-lyase (METase) enzyme, by biological methylation of selenide ion or in vivo by reduction of methaneseleninic acid (CH3SeO2H), has been invoked to explain the anticancer activity of certain organoselenium compounds.[2][3][4] Precursors of methaneselenol are under active investigation in cancer prevention and therapy. In these studies, methaneselenol is found to be more biologically active than ethaneselenol or 2-propaneselenol.[5]
Preparation
Selenols are prepared usually by the reaction of organolithium reagents or Grignard reagents with elemental Se. For example, benzeneselenol is generated by the reaction of phenylmagnesium bromide with selenium followed by acidicifation:[6]
Another preparative route to selenols involves the alkylation of selenourea, followed by hydrolysis. Selenols are often generated by reduction of diselenides followed by protonation of the resulting selenoate:
- 2 RSeSeR + 2 LiHB(C2H5)3 → 2 RSeLi + 2 B(C2H5)3 + H2
- RSeLi + HCl → RSeH + LiCl
Dimethyl diselenide can be easily reduced to methaneselenol within cells.[7]
Reactions
Selenols are easily oxidized to diselenides, compounds containing an Se-Se bond. For example treatment of benzeneselenol with bromine gives diphenyl diselenide.
- 2 C6H5SeH + Br2 → (C6H5Se)2 + 2 HBr
Safety
Organoselenium compounds (or any selenium compound) are cumulative poisons despite the fact that trace amounts of Se are required for health.
References
- 1 2 Wessjohann, L.A.; Schneider, A.; Abbas, M.; Brandt, W. "Selenium in Chemistry and Biochemistry in Comparison to Sulfur" Biol. Chem., Vol. 388, pp. 997–1006, 2007. doi:10.1515/BC.2007.138
- ↑ Zeng, H.; Briske-Anderson, M.; Wu, M.; Moyer, M. P. Methylselenol, a selenium metabolite, plays common and different roles in cancerous colon HCT116 cell and noncancerous NCM460 colon cell proliferation, Nutrition and Cancer 2012, 64, 128–135. doi: 10.1080/01635581.2012.630555
- ↑ Fernandes, A.P.; Wallenberg, M.; Gandin, V.; Misra1, S.; Marzano, C.; Rigobello, M. P.; Kumar, S.; Björnstedt, M. "Methylselenol formed by spontaneous methylation of selenide is a superior selenium substrate to the thioredoxin and glutaredoxin systems", PLoS ONE 2012, 7, e50727. doi:10.1371/journal.pone.0050727
- ↑ Ip, C.; Dong, Y.; Ganther, H. E. "New concepts in selenium chemoprevention". Cancer Metastasis Rev. 2002, 21, 281–289.
- ↑ Zuazo, A.; Plano, D.; Ansó, E.; Lizarraga, E.; Font, M.; Irujo, J.J.M. "Cytotoxic and proapototic activities of imidoselenocarbamate derivatives are dependent on the release of methylselenol", Chem. Res. Toxicol. 2012, 25, 2479–2489. doi:10.1021/tx300306t
- ↑ Foster, D. G. (1955). "Selenophenol". Org. Synth.; Coll. Vol. 3, p. 771
- ↑ Gabel-Jensen, C.; Lunoe, K.; Gammelgaard, B. "Formation of methylselenol, dimethylselenide and dimethyldiselenide in in vitro metabolism models determined by headspace GC-MS." Metallomics 2010, 2, 167–173.
