Methoxphenidine
 | |
| Systematic (IUPAC) name | |
|---|---|
|
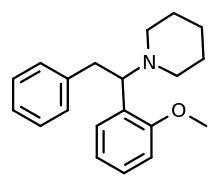
(±)-1-[1-(2-methoxyphenyl)-2-phenylethyl]piperidine | |
| Clinical data | |
| Legal status |
|
| Routes of administration | Oral, Rectal |
| Identifiers | |
| CAS Number |
127529-46-8 |
| PubChem | CID 67833251 |
| Chemical data | |
| Formula | C20H25NO |
| Molar mass | 295.4186 g/mol |
| |
| |
Methoxphenidine (methoxydiphenidine, 2-MeO-Diphenidine, MXP) is a dissociative of the diarylethylamine class that has been sold online as a designer drug.[1][2] Methoxphenidine was first reported in a 1989 patent where it was tested as a treatment for neurotoxic injury.[3] Shortly after the 2013 UK ban on arylcyclohexylamines methoxphenidine and the related compound diphenidine became available on the gray market, where it has been encountered as a powder and in tablet form.[4] Though diphenidine possesses higher affinity for the NMDA receptor, anecdotal reports suggest methoxphenidine has greater oral potency.[1] Of the three isomeric anisyl-substituents methoxphenidine has affinity for the NMDA receptor that is higher than 4-MeO-Diphenidine but lower than 3-MeO-Diphenidine,[3] a structure–activity relationship shared by the anisylcyclohexylamines.
Side effects
Acute methoxphenidine intoxication has been reported to produce confusion, hypertension, and tachycardia that was responsive to treatment with intravenous lorazepam,[5][6] methoxphenidine has also been associated with three published fatalities[7] and one case of impaired driving.[8]
Legal Status
As of October 2015 MXP is a controlled substance in China.[9]
MXP is also banned in Sweden.[10]
See also
- AD-1211
- Diphenidine
- Ephenidine
- Fluorolintane
- Lanicemine
- Lefetamine
- MT-45
- NMDA receptor antagonist
- Phencyclidine
References
- 1 2 Morris, H.; Wallach, J. (2014). "From PCP to MXE: a comprehensive review of the non-medical use of dissociative drugs". Drug Testing and Analysis 6: 614–632. doi:10.1002/dta.1620. PMID 24678061.
- ↑ Marie Claire Van Hout, Evelyn Hearne (2015). ""Word of Mouse": Indigenous Harm Reduction and Online Consumerism of the Synthetic Compound Methoxphenidine". Journal of Psychoactive Drugs 47 (1): 30–41. doi:10.1080/02791072.2014.974002. PMID 25715070.
- 1 2 Nancy M. Gray, Brian K. Cheng. "Patent EP 0346791 B1 - 1,2-diarylethylamines for treatment of neurotoxic injury". Retrieved 17 June 2015.
- ↑ McLaughlin, G.; Morris, N.; Kavanagh, P.; Power, J.; O'Brien, J.; Talbot, B.; Elliott, S.; Wallach, J.; Hoang, K.; Morris, H.; Brandt, S. (2015). "Test purchase, synthesis, and characterization of 2-methoxydiphenidine (MXP) and differentiation from its meta- and para-substituted isomers". Drug Testing and Analysis. doi:10.1002/dta.1800.
- ↑ Hofer, K.E.; Degrandi, C.; Müller, D.M.; Zürrer-Härdi, U.; Wahl, S.; Rauber-Lüthy, C.; Ceschi, A. (2014). "Acute toxicity associated with the recreational use of the novel dissociative psychoactive substance methoxphenidine". Clinical Toxicology 52: 1288–1291. doi:10.3109/15563650.2014.974264.
- ↑ Anders Helander, Olof Beck, Matilda Bäckberg (April 2015). "Intoxications by the dissociative new psychoactive substances diphenidine and methoxphenidine". Clinical Toxicology 53 (5): 446–453. doi:10.3109/15563650.2015.1033630. PMID 25881797.
- ↑ Elliott, S.P.; Brandt, S.D.; Wallach, J.; Morris, H.; Kavanagh, S. (2015). "First Reported Fatalities Associated with the ‘Research Chemical’ 2-Methoxydiphenidine". Analytical Toxicology.
- ↑ Nicole Stachel, Andrea Jacobsen-Bauer, Gisela Skopp (October 2015). "A methoxydiphenidine-impaired driver". International Journal of Legal Medicine. doi:10.1007/s00414-015-1280-5.
- ↑ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Retrieved 1 October 2015.
- ↑ "Fler ämnen föreslås bli klassade som narkotika eller hälsofarlig vara" (in Swedish). Folkhälsomyndigheten. 24 March 2015. Retrieved 21 October 2015.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||