Chromatophore

Chromatophores are pigment-containing and light-reflecting cells, or groups of cells, found in a wide range of animals including amphibians, fish, reptiles, crustaceans and cephalopods. Mammals and birds, in contrast, have a class of cells called melanocytes for coloration.
Chromatophores are largely responsible for generating skin and eye colour in cold-blooded animals and are generated in the neural crest during embryonic development. Mature chromatophores are grouped into subclasses based on their colour (more properly "hue") under white light: xanthophores (yellow), erythrophores (red), iridophores (reflective / iridescent), leucophores (white), melanophores (black/brown), and cyanophores (blue).

Some species can rapidly change colour through mechanisms that translocate pigment and reorient reflective plates within chromatophores. This process, often used as a type of camouflage, is called physiological colour change or metachrosis.[1] Cephalopods such as the octopus have complex chromatophore organs controlled by muscles to achieve this, whereas vertebrates such as chameleons generate a similar effect by cell signalling. Such signals can be hormones or neurotransmitters and may be initiated by changes in mood, temperature, stress or visible changes in the local environment. Chromatophores are studied by scientists to understand human disease and as a tool in drug discovery.
Human discovery
Aristotle mentioned the ability of the octopus to change colour for both camouflage and signalling in his Historia animalium (ca 400 BC):[2]
The octopus ... seeks its prey by so changing its colour as to render it like the colour of the stones adjacent to it; it does so also when alarmed.
Giosuè Sangiovanni was the first to describe invertebrate pigment-bearing cells as cromoforo in an Italian science journal in 1819.[3]
Charles Darwin described the colour-changing abilities of the cuttlefish in The Voyage of the Beagle (1860):[4]
These animals also escape detection by a very extraordinary, chameleon-like power of changing their colour. They appear to vary their tints according to the nature of the ground over which they pass: when in deep water, their general shade was brownish purple, but when placed on the land, or in shallow water, this dark tint changed into one of a yellowish green. The colour, examined more carefully, was a French grey, with numerous minute spots of bright yellow: the former of these varied in intensity; the latter entirely disappeared and appeared again by turns. These changes were effected in such a manner that clouds, varying in tint between a hyacinth red and a chestnut-brown, were continually passing over the body. Any part, being subjected to a slight shock of galvanism, became almost black: a similar effect, but in a less degree, was produced by scratching the skin with a needle. These clouds, or blushes as they may be called, are said to be produced by the alternate expansion and contraction of minute vesicles containing variously coloured fluids.
Classification
The term chromatophore was adopted (following Sangiovanni's chromoforo) as the name for pigment-bearing cells derived from the neural crest of cold-blooded vertebrates and cephalopods. The word itself comes from the Greek words khrōma (χρωμα) meaning "colour," and phoros (φορος) meaning "bearing". In contrast, the word chromatocyte (cyte or κυτε being Greek for "cell") was adopted for the cells responsible for colour found in birds and mammals. Only one such cell type, the melanocyte, has been identified in these animals.
It was only in the 1960s that chromatophores were well enough understood to enable them to be classified based on their appearance. This classification system persists to this day, even though the biochemistry of the pigments may be more useful to a scientific understanding of how the cells function.[5]
Colour-producing molecules fall into two distinct classes: biochromes and structural colours or "schemochromes".[6] The biochromes include true pigments, such as carotenoids and pteridines. These pigments selectively absorb parts of the visible light spectrum that makes up white light while permitting other wavelengths to reach the eye of the observer. Structural colours are produced by various combinations of diffraction, reflection or scattering of light from structures with a scale around a quarter of the wavelength of light. Many such structures interfere with some wavelengths (colours) of light and transmit others, simply because of their scale, so they often produce iridescence, creating different colours when seen from different directions.
Whereas all chromatophores contain pigments or reflecting structures (except when there has been a mutation, as in albinism), not all pigment-containing cells are chromatophores. Haem, for example, is a biochrome responsible for the red appearance of blood. It is found primarily in red blood cells (erythrocytes), which are generated in bone marrow throughout the life of an organism, rather than being formed during embryological development. Therefore, erythrocytes are not classified as chromatophores.

Xanthophores and erythrophores
Chromatophores that contain large amounts of yellow pteridine pigments are named xanthophores; those with mainly red/orange carotenoids are termed erythrophores.[5] However, vesicles containing pteridine and carotenoids are sometimes found in the same cell, in which case the overall colour depends on the ratio of red and yellow pigments.[7] Therefore, the distinction between these chromatophore types is not always clear.
Most chromatophores can generate pteridines from guanosine triphosphate, but xanthophores appear to have supplemental biochemical pathways enabling them to accumulate yellow pigment. In contrast, carotenoids are metabolised and transported to erythrophores. This was first demonstrated by rearing normally green frogs on a diet of carotene-restricted crickets. The absence of carotene in the frogs' diet meant that the red/orange carotenoid colour 'filter' was not present in their erythrophores. This made the frogs appear blue instead of green.[8]
Iridophores and leucophores
Iridophores, sometimes also called guanophores, are pigment cells that reflect light using plates of crystalline chemochromes made from guanine.[9] When illuminated they generate iridescent colours because of the diffraction of light within the stacked plates. Orientation of the schemochrome determines the nature of the colour observed.[10] By using biochromes as coloured filters, iridophores create an optical effect known as Tyndall or Rayleigh scattering, producing bright-blue or -green colours.[11]
A related type of chromatophore, the leucophore, is found in some fish, in particular in the tapetum lucidum. Like iridophores, they utilize crystalline purines (often guanine) to reflect light. Unlike iridophores, however, leucophores have more organized crystals that reduce diffraction. Given a source of white light, they produce a white shine. As with xanthophores and erythrophores, in fish the distinction between iridophores and leucophores is not always obvious, but, in general, iridophores are considered to generate iridescent or metallic colours, whereas leucophores produce reflective white hues.[11]
Melanophores

Melanophores contain eumelanin, a type of melanin, that appears black or dark-brown because of its light absorbing qualities. It is packaged in vesicles called melanosomes and distributed throughout the cell. Eumelanin is generated from tyrosine in a series of catalysed chemical reactions. It is a complex chemical containing units of dihydroxyindole and dihydroxyindole-2-carboxylic acid with some pyrrole rings.[12] The key enzyme in melanin synthesis is tyrosinase. When this protein is defective, no melanin can be generated resulting in certain types of albinism. In some amphibian species there are other pigments packaged alongside eumelanin. For example, a novel deep (wine) red-colour pigment was identified in the melanophores of phyllomedusine frogs.[13] This was subsequently identified as pterorhodin, a pteridine dimer that accumulates around eumelanin core, and it is also present in a variety of tree frog species from Australia and Papua New Guinea. While it is likely that other lesser-studied species have complex melanophore pigments, it is nevertheless true that the majority of melanophores studied to date do contain eumelanin exclusively.[14]
Humans have only one class of pigment cell, the mammalian equivalent of melanophores, to generate skin, hair, and eye colour. For this reason, and because the large number and contrasting colour of the cells usually make them very easy to visualise, melanophores are by far the most widely studied chromatophore. However, there are differences between the biology of melanophores and that of melanocytes. In addition to eumelanin, melanocytes can generate a yellow/red pigment called phaeomelanin.
Cyanophores
Nearly all the vibrant blues in animals and plants are created by structural coloration rather than by pigments. However, some types of mandarinfish do possess vesicles of a cyan biochrome of unknown chemical structure in cells named cyanophores.[11] Although they appear unusual in their limited taxonomic range, there may be cyanophores (as well as further unusual chromatophore types) in other fish and amphibians. For example, brightly coloured chromatophores with undefined pigments are found in both poison dart frogs and glass frogs,[15] and atypical dichromatic chromatophores, named erythro-iridophores have been described in Pseudochromis diadema.[16]
Pigment translocation
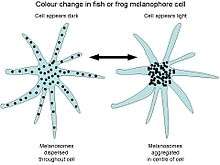
Many species are able to translocate the pigment inside their chromatophores, resulting in an apparent change in body colour. This process, known as physiological colour change, is most widely studied in melanophores, since melanin is the darkest and most visible pigment. In most species with a relatively thin dermis, the dermal melanophores tend to be flat and cover a large surface area. However, in animals with thick dermal layers, such as adult reptiles, dermal melanophores often form three-dimensional units with other chromatophores. These dermal chromatophore units (DCU) consist of an uppermost xanthophore or erythrophore layer, then an iridophore layer, and finally a basket-like melanophore layer with processes covering the iridophores.[17]
Both types of melanophore are important in physiological colour change. Flat dermal melanophores often overlay other chromatophores, so when the pigment is dispersed throughout the cell the skin appears dark. When the pigment is aggregated toward the centre of the cell, the pigments in other chromatophores are exposed to light and the skin takes on their hue. Likewise, after melanin aggregation in DCUs, the skin appears green through xanthophore (yellow) filtering of scattered light from the iridophore layer. On the dispersion of melanin, the light is no longer scattered and the skin appears dark. As the other biochromatic chromatophores are also capable of pigment translocation, animals with multiple chromatophore types can generate a spectacular array of skin colours by making good use of the divisional effect.[18][19]
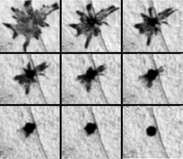
The control and mechanics of rapid pigment translocation has been well studied in a number of different species, in particular amphibians and teleost fish.[11][20] It has been demonstrated that the process can be under hormonal or neuronal control or both. Neurochemicals that are known to translocate pigment include noradrenaline, through its receptor on the surface on melanophores.[21] The primary hormones involved in regulating translocation appear to be the melanocortins, melatonin, and melanin-concentrating hormone (MCH), that are produced mainly in the pituitary, pineal gland, and hypothalamus, respectively. These hormones may also be generated in a paracrine fashion by cells in the skin. At the surface of the melanophore, the hormones have been shown to activate specific G-protein-coupled receptors that, in turn, transduce the signal into the cell. Melanocortins result in the dispersion of pigment, while melatonin and MCH results in aggregation.[22]
Numerous melanocortin, MCH and melatonin receptors have been identified in fish[23] and frogs,[24] including a homologue of MC1R,[25] a melanocortin receptor known to regulate skin and hair colour in humans.[26] It has been demonstrated that MC1R is required in zebrafish for dispersion of melanin.[27] Inside the cell, cyclic adenosine monophosphate (cAMP) has been shown to be an important second messenger of pigment translocation. Through a mechanism not yet fully understood, cAMP influences other proteins such as protein kinase A to drive molecular motors carrying pigment containing vesicles along both microtubules and microfilaments.[28][29][30]
Background adaptation

Most fish, reptiles and amphibians undergo a limited physiological colour change in response to a change in environment. This type of camouflage, known as background adaptation, most commonly appears as a slight darkening or lightening of skin tone to approximately mimic the hue of the immediate environment. It has been demonstrated that the background adaptation process is vision-dependent (it appears the animal needs to be able to see the environment to adapt to it),[31] and that melanin translocation in melanophores is the major factor in colour change.[22] Some animals, such as chameleons and anoles, have a highly developed background adaptation response capable of generating a number of different colours very rapidly. They have adapted the capability to change colour in response to temperature, mood, stress levels, and social cues, rather than to simply mimic their environment.
Development
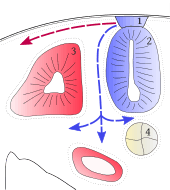
During vertebrate embryonic development, chromatophores are one of a number of cell types generated in the neural crest, a paired strip of cells arising at the margins of the neural tube. These cells have the ability to migrate long distances, allowing chromatophores to populate many organs of the body, including the skin, eye, ear, and brain. Leaving the neural crest in waves, chromatophores take either a dorsolateral route through the dermis, entering the ectoderm through small holes in the basal lamina, or a ventromedial route between the somites and the neural tube. The exception to this is the melanophores of the retinal pigmented epithelium of the eye. These are not derived from the neural crest. Instead, an outpouching of the neural tube generates the optic cup, which, in turn, forms the retina.
When and how multipotent chromatophore precursor cells (called chromatoblasts) develop into their daughter subtypes is an area of ongoing research. It is known in zebrafish embryos, for example, that by 3 days after fertilization each of the cell classes found in the adult fish—melanophores, xanthophores and iridophores—are already present. Studies using mutant fish have demonstrated that transcription factors such as kit, sox10, and mitf are important in controlling chromatophore differentiation.[32] If these proteins are defective, chromatophores may be regionally or entirely absent, resulting in a leucistic disorder.
Practical applications
Chromatophores are sometimes used in applied research. For example, zebrafish larvae are used to study how chromatophores organise and communicate to accurately generate the regular horizontal striped pattern as seen in adult fish.[33] This is seen as a useful model system for understanding patterning in the evolutionary developmental biology field. Chromatophore biology has also been used to model human condition or disease, including melanoma and albinism. Recently, the gene responsible for the melanophore-specific golden zebrafish strain, Slc24a5, was shown to have a human equivalent that strongly correlates with skin colour.[34]
Chromatophores are also used as a biomarker of blindness in cold-blooded species, as animals with certain visual defects fail to background adapt to light environments.[31] Human homologues of receptors that mediate pigment translocation in melanophores are thought to be involved in processes such as appetite suppression and tanning, making them attractive targets for drugs.[25] Therefore, pharmaceutical companies have developed a biological assay for rapidly identifying potential bioactive compounds using melanophores from the African clawed frog.[35] Other scientists have developed techniques for using melanophores as biosensors,[36] and for rapid disease detection (based on the discovery that pertussis toxin blocks pigment aggregation in fish melanophores).[37] Potential military applications of chromatophore-mediated colour changes have been proposed, mainly as a type of active camouflage, which could as in cuttlefish make objects nearly invisible.[38][39]
Cephalopod chromatophores

Coleoid cephalopods (including octopuses, squids and cuttlefish) have complex multicellular organs that they use to change colour rapidly, producing a wide variety of bright colours and patterns. Each chromatophore unit is composed of a single chromatophore cell and numerous muscle, nerve, glial, and sheath cells.[40] Inside the chromatophore cell, pigment granules are enclosed in an elastic sac, called the cytoelastic sacculus. To change colour the animal distorts the sacculus form or size by muscular contraction, changing its translucency, reflectivity, or opacity. This differs from the mechanism used in fish, amphibians, and reptiles in that the shape of the sacculus is changed, rather than translocating pigment vesicles within the cell. However, a similar effect is achieved.
Octopuses (and most cuttlefish) can operate chromatophores in complex, wavelike chromatic displays, resulting in a variety of rapidly changing colour schemes. The nerves that operate the chromatophores are thought to be positioned in the brain in a pattern similar to that of the chromatophores they each control. This means the pattern of colour change matches the pattern of neuronal activation. This may explain why, as the neurons are activated one after another, the colour change occurs in waves.[41] Like chameleons, cephalopods use physiological colour change for social interaction. They are also among the most skilled at background adaptation, having the ability to match both the colour and the texture of their local environment with remarkable accuracy.
See also
Notes
- ↑ Scott M. Boback & Lynn M. Siefferman (2010). "Variation in Color and Color Change in Island and Mainland Boas (Boa constrictor)". Journal of Herpetology 44 (4): 506–515. doi:10.1670/09-026.1.
- ↑ Aristotle. Historia Animalium. IX, 622a: 2-10. About 400 BC. Cited in Luciana Borrelli, Francesca Gherardi, Graziano Fiorito. A catalogue of body patterning in Cephalopoda. Firenze University Press, 2006. Abstract Google books
- ↑ Sangiovanni G. Descrizione di un particolare sistema di organi cromoforo espansivo-dermoideo e dei fenomeni che esso produce, scoperto nei molluschi cefaloso. G. Enciclopedico Napoli. 1819; 9:1–13.
- ↑ Darwin, Charles (1860). "Chapter 1. Habits of a Sea-slug and Cuttle-fish". Journal Of Researches Into The Natural History And Geology Of The Countries Visited During The Voyage Round The World Of H.M.S. 'Beagle' Under The Command Of Captain Fitz Roy, R.N. John Murray, London. p. 7.
- 1 2 Bagnara, JT (1966). "Cytology and cytophysiology of non-melanophore pigment cells". International Review of Cytology. International Review of Cytology 20: 173–205. doi:10.1016/S0074-7696(08)60801-3. ISBN 978-0-12-364320-9. PMID 5337298.
- ↑ Fox, DL. Animal Biochromes and Structural Colors: Physical, Chemical, Distributional & Physiological Features of Colored Bodies in the Animal World. University of California Press, Berkeley, 1976. ISBN 0-520-02347-1
- ↑ Matsumoto, J (1965). "Studies on fine structure and cytochemical properties of erythrophores in swordtail, Xiphophorus helleri, with special reference to their pigment granules (pterinosomes)". J Cell Biol 27 (3): 493–504. doi:10.1083/jcb.27.3.493. PMC 2106771. PMID 5885426.
- ↑ Bagnara JT. Comparative Anatomy and Physiology of Pigment Cells in Nonmammalian Tissues. In: The Pigmentary System: Physiology and Pathophysiology, Oxford University Press, 1998. ISBN 0-19-509861-7
- ↑ Taylor, JD. (1969). "The effects of intermedin on the ultrastructure of amphibian iridophores". Gen Comp Endocrinol 12 (3): 405–16. doi:10.1016/0016-6480(69)90157-9. PMID 5769930.
- ↑ Morrison, RL. (1995). "A transmission electron microscopic (TEM) method for determining structural colors reflected by lizard iridophores". Pigment Cell Res 8 (1): 28–36. doi:10.1111/j.1600-0749.1995.tb00771.x. PMID 7792252.
- 1 2 3 4 Fujii, R. (2000). "The regulation of motile activity in fish chromatophores". Pigment Cell Res 13 (5): 300–19. doi:10.1034/j.1600-0749.2000.130502.x. PMID 11041206.
- ↑ Ito, S; Wakamatsu, K. (2003). "Quantitative analysis of eumelanin and pheomelanin in humans, mice, and other animals: a comparative review". Pigment Cell Res 16 (5): 523–31. doi:10.1034/j.1600-0749.2003.00072.x. PMID 12950732.
- ↑ Bagnara, J.T.; Taylor, JD; Prota, G (1973). "Color changes, unusual melanosomes, and a new pigment from leaf frogs". Science 182 (4116): 1034–5. doi:10.1126/science.182.4116.1034. PMID 4748673.
- ↑ Bagnara, J.T. (2003). "Enigmas of Pterorhodin, a Red Melanosomal Pigment of Tree Frogs". Pigment Cell Research 16 (5): 510–516. doi:10.1034/j.1600-0749.2003.00075.x. PMID 12950730.
- ↑ Schwalm, PA; Starrett, PH; McDiarmid, RW (1977). "Infrared reflectance in leaf-sitting neotropical frogs". Science 196 (4295): 1225–7. doi:10.1126/science.860137. PMID 860137.
- ↑ Goda M, Ohata M, Ikoma H, Fujiyoshi Y, Sugimoto M, Fujii R (2011). "Integumental reddish-violet coloration owing to novel dichromatic chromatophores in the teleost fish, Pseudochromis diadema". Pigment Cell Melanoma Res 24 (4): 614–7. doi:10.1111/j.1755-148X.2011.00861.x. PMID 21501419.
- ↑ Bagnara, JT; Taylor, JD; Hadley, ME (1968). "The dermal chromatophore unit". J Cell Biol 38 (1): 67–79. doi:10.1083/jcb.38.1.67. PMC 2107474. PMID 5691979.
- ↑ Palazzo, RE; Lynch, TJ; Lo, SJ; Taylor, JD; Tchen, TT (1989). "Rearrangements of pterinosomes and cytoskeleton accompanying pigment dispersion in goldfish xanthophores". Cell Motil Cytoskeleton 13 (1): 9–20. doi:10.1002/cm.970130103. PMID 2543509.
- ↑ Porras, MG; De Loof, A; Breuer, M; Aréchiga, H (2003). "Procambarus clarkii". Peptides 24 (10): 1581–9. doi:10.1016/j.peptides.2003.08.016. PMID 14706537.
- ↑ Deacon, SW; Serpinskaya, AS; Vaughan, PS; Lopez Fanarraga, M; Vernos, I; Vaughan, KT; Gelfand, VI (2003). "Dynactin is required for bidirectional organelle transport". The Journal of Cell Biology 160 (3): 297–301. doi:10.1083/jcb.200210066. PMC 2172679. PMID 12551954.
- ↑ Aspengren, S; Sköld, HN; Quiroga, G; Mårtensson, L; Wallin, M (2003). "Noradrenaline- and melatonin-mediated regulation of pigment aggregation in fish melanophores". Pigment Cell Res 16 (1): 59–64. doi:10.1034/j.1600-0749.2003.00003.x. PMID 12519126.
- 1 2 Logan, D. W.; Burn, SF; Jackson, IJ (2006). "Regulation of pigmentation in zebrafish melanophores". Pigment Cell Research 19 (3): 206–213. doi:10.1111/j.1600-0749.2006.00307.x. PMID 16704454.
- ↑ Logan, DW; Bryson-Richardson, RJ; Taylor, MS; Currie, P; Jackson, IJ (2003). "Sequence characterization of teleost fish melanocortin receptors". Ann N Y Acad Sci 994: 319–30. doi:10.1111/j.1749-6632.2003.tb03196.x. PMID 12851332.
- ↑ Sugden, D; Davidson, K; Hough, KA; Teh, MT (2004). "Melatonin, melatonin receptors and melanophores: a moving story". Pigment Cell Res 17 (5): 454–60. doi:10.1111/j.1600-0749.2004.00185.x. PMID 15357831.
- 1 2 Logan, DW; Bryson-Richardson, RJ; Pagán, KE; Taylor, MS; Currie, PD; Jackson, IJ (2003). "The structure and evolution of the melanocortin and MCH receptors in fish and mammals". Genomics 81 (2): 184–91. doi:10.1016/S0888-7543(02)00037-X. PMID 12620396.
- ↑ Valverde, P; Healy, E; Jackson, I; Rees, JL; Thody, AJ (1995). "Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans". Nat Genet 11 (3): 328–30. doi:10.1038/ng1195-328. PMID 7581459.
- ↑ Richardson, J; Lundegaard, PR; Reynolds, NL; Dorin, JR; Porteous, DJ; Jackson, IJ; Patton, EE (2008). "mc1r Pathway regulation of zebrafish melanosome dispersion". Zebrafish 5 (4): 289–95. doi:10.1089/zeb.2008.0541. PMID 19133827.
- ↑ Snider, J; Lin, F; Zahedi, N; Rodionov, V; Yu, CC; Gross, SP (2004). "Intracellular actin-based transport: How far you go depends on how often you switch". Proceedings of the National Academy of Sciences of the United States of America 101 (36): 13204–9. doi:10.1073/pnas.0403092101. PMC 516548. PMID 15331778.
- ↑ Rodionov, VI; Hope, AJ; Svitkina, TM; Borisy, GG (1998). "Functional coordination of microtubule-based and actin-based motility in melanophores". Current biology : CB 8 (3): 165–8. doi:10.1016/S0960-9822(98)70064-8. PMID 9443917.
- ↑ Kashina, AS; Semenova, IV; Ivanov, PA; Potekhina, ES; Zaliapin, I; Rodionov, VI (2004). "Protein kinase A, which regulates intracellular transport, forms complexes with molecular motors on organelles". Current biology : CB 14 (20): 1877–81. doi:10.1016/j.cub.2004.10.003. PMID 15498498.
- 1 2 Neuhauss, SC. (2003). "Behavioral genetic approaches to visual system development and function in zebrafish" (PDF). J Neurobiol 54 (1): 148–60. doi:10.1002/neu.10165. PMID 12486702.
- ↑ Kelsh, RN; Schmid, B; Eisen, JS (2000). "Genetic analysis of melanophore development in zebrafish embryos". Dev Biol 225 (2): 277–93. doi:10.1006/dbio.2000.9840. PMID 10985850.
- ↑ Kelsh, RN (2004). "Genetics and evolution of pigment patterns in fish". Pigment cell research / sponsored by the European Society for Pigment Cell Research and the International Pigment Cell Society 17 (4): 326–36. doi:10.1111/j.1600-0749.2004.00174.x. PMID 15250934.
- ↑ Lamason, RL; Mohideen, MA; Mest, JR; Wong, AC; Norton, HL; Aros, MC; Jurynec, MJ; Mao, X; et al. (2005). "SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans". Science 310 (5755): 1782–6. doi:10.1126/science.1116238. PMID 16357253.
- ↑ Jayawickreme, CK; Sauls, H; Bolio, N; Ruan, J; Moyer, M; Burkhart, W; Marron, B; Rimele, T; Shaffer, J (1999). "Use of a cell-based, lawn format assay to rapidly screen a 442,368 bead-based peptide library". J Pharmacol Toxicol Methods 42 (4): 189–97. doi:10.1016/S1056-8719(00)00083-6. PMID 11033434.
- ↑ Andersson, TP; Filippini, D; Suska, A; Johansson, TL; Svensson, SP; Lundström, I (2005). "Frog melanophores cultured on fluorescent microbeads: biomimic-based biosensing". Biosens Bioelectron 21 (1): 111–20. doi:10.1016/j.bios.2004.08.043. PMID 15967358.
- ↑ Karlsson, JO; Andersson, RG; Askelöf, P; Elwing, H; Granström, M; Grundström, N; Lundström, I; Ohman, L (1991). "The melanophore aggregating response of isolated fish scales: a very rapid and sensitive diagnosis of whooping cough". FEMS Microbiol Lett 66 (2): 169–75. PMID 1936946.
- ↑ Hansford, Dave (August 6, 2008). "Cuttlefish Change Color, Shape-Shift to Elude Predators". National Geographic News (Wellington, New Zealand).
[...] cuttlefish have instead relied on invisibility, a talent that may have applications for human technology. Norman said the military has shown interest in cuttlefish camouflage with a view to one day incorporating similar mechanisms in soldiers' uniforms.
- ↑ Lee I. Nanotubes for noisy signal processing PhD Thesis. 2005; University of Southern California.
- ↑ Cloney, RA; Florey, E (1968). "Ultrastructure of cephalopod chromatophore organs". Zeitschrift für Zellforschung und mikroskopische Anatomie 89 (2): 250–80. doi:10.1007/BF00347297. PMID 5700268.
- ↑ Demski, LS (1992). "Chromatophore systems in teleosts and cephalopods: a levels oriented analysis of convergent systems". Brain, behavior and evolution 40 (2–3): 141–56. doi:10.1159/000113909. PMID 1422807.
External links
- Nature's Palette - how animals produce colour PDF (1.20 MB)
- Video footage of octopus background adaptation
- Video footage of squid chromatophore patterning
- Tree of Life Web Project: Cephalopod Chromatophores
| ||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
.jpg)


