Pelagic fish

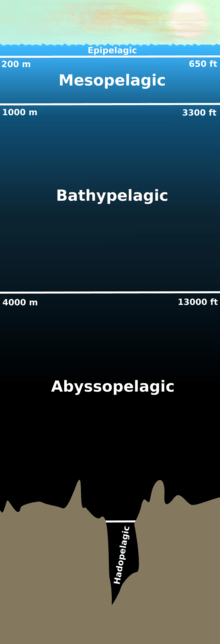
Pelagic fish live in the pelagic zone of ocean or lake waters – being neither close to the bottom nor near the shore – in contrast with demersal fish, which do live on or near the bottom, and reef fish, which are associated with coral reefs.[1]
The marine pelagic environment is the largest aquatic habitat on Earth, occupying 1,370 million cubic kilometres (330 million cubic miles), and is the habitat for 11% of known fish species. The oceans have a mean depth of 4000 metres. About 98% of the total water volume is below 100 metres (330 ft), and 75% is below 1,000 metres (3,300 ft).[2]
Marine pelagic fish can be divided into pelagic coastal fish and oceanic pelagic fish.[3] Coastal fish inhabit the relatively shallow and sunlit waters above the continental shelf, while oceanic fish (which may well also swim inshore) inhabit the vast and deep waters beyond the continental shelf.[4]
Pelagic fish range in size from small coastal forage fish, such as herrings and sardines, to large apex predator oceanic fishes, such as bluefin tuna and oceanic sharks.[1] They are usually agile swimmers with streamlined bodies, capable of sustained cruising on long-distance migrations. The Indo-Pacific sailfish, an oceanic pelagic fish, can sprint at over 110 kilometres per hour. Some tuna species cruise across the Pacific Ocean. Many pelagic fish swim in schools weighing hundreds of tonnes. Others are solitary, like the large ocean sunfish weighing over 500 kilograms, which sometimes drift passively with ocean currents, eating jellyfish.[1]
Epipelagic fish


Epipelagic fish inhabit the epipelagic zone. The epipelagic zone is the water from the surface of the sea down to 200 metres. It is also referred to as the surface waters or the sunlit zone, and includes the photic zone. The photic zone is defined as the surface waters down to the point where the sunlight has attenuated to 1% of the surface value. This depth depends on how turbid the water is, but in clear water can extend to 200 metres, coinciding with the epipelagic zone. The photic zone has sufficient light for phytoplankton to photosynthese.[5]
The epipelagic zone is vast, and is the home for most pelagic fish. The zone is well lit so visual predators can use their eyesight, is usually well mixed and oxygenated from wave action, and can be a good habitat for algae to grow. However, it is an almost featureless habitat. This lack of habitat diversity results in a lack of species diversity, so the zone supports less than 2% of the world's known fish species. Much of the zone lacks nutrients for supporting fish, so epipelagic fish tend to be found in coastal water above the continental shelves, where land runoff can provide nutrients, or in those parts of the ocean where upwelling moves nutrients into the area.[5]
Epipelagic fish can be broadly divided into small forage fish and larger predator fish which feed on them. Forage fish school and filter feed on plankton. Most epipelagic fish have streamlined bodies capable of sustained cruising on migrations. In general, predatory and forage fish share the same morphological features. Predator fish are usually fusiform with large mouths, smooth bodies, and deeply forked tails. Many use vision to predate zooplankton or smaller fish, while others filter feed on plankton.
Most epipelagic predator fish and their smaller prey fish are countershaded with silvery colours which reduce visibility by scattering incoming light.[5] The silvering is achieved with reflective fish scales that function as small mirrors. This can give an effect of transparency. At medium depths at sea, light comes from above, so a mirror oriented vertically makes animals such as fish invisible from the side.[6]
In the shallower epipelagic waters, the mirrors must reflect a mixture of wavelengths, and the fish accordingly has crystal stacks with a range of different spacings. A further complication for fish with bodies that are rounded in cross-section is that the mirrors would be ineffective if laid flat on the skin, as they would fail to reflect horizontally. The overall mirror effect is achieved with many small reflectors, all oriented vertically.[6]
Though the number of species is limited, epipelagic fishes are abundant. What they lack in diversity they make up in numbers. Forage fish occur in huge numbers, and large fish that prey on them are often sought after as premier food fish. As a group, epipelagic fishes form the most valuable fisheries in the world.[5]
Many forage fish are facultative predators that can pick individual copepods or fish larvae out of the water column, and then change to filter feeding on phytoplankton when energetically that gives better results. Filter feeding fish usually use long fine gill rakers to strain small organisms from the water column. Some of the largest epipelagic fishes, such as the basking shark and whale shark are filter feeders, and so are some of the smallest, such as adult sprats and anchovies.[7]
Ocean waters that are exceptionally clear contain little food. Areas of high productivity tend to be somewhat turbid from plankton blooms. These attract the filter feeding plankton eaters, which in turn attract the higher predators. Tuna fishing tends to be optimum when water turbidity, measured by the maximum depth a secchi disc can be seen during a sunny day, is 15 to 35 metres.[8]
Floating objects




Epipelagic fish are fascinated with floating objects. They aggregate in considerable numbers around objects such as drifting flotsam, rafts, jellyfish and floating seaweed. The objects appear to provide a "visual stimulus in an optical void".[9] Floating objects can offer some protection for juvenile fish from predators. The availability of lots of drifting seaweed or jellyfish can result in significant increases in the survival rates of some juvenile species.[10]
Many coastal juveniles use seaweed for the shelter and the food that is available from invertebrates and other fish associated with it. Drifting seaweed, particularly the pelagic Sargassum, provide a niche habitat with its own shelter and food, and even supports its own unique fauna, such as the sargassum fish.[7] One study, off Florida, found 54 species from 23 families living in flotsam from Sargassum mats.[11] Jellyfish are also used by juvenile fish for shelter and food, even though jellyfish may prey on small fish.[12]
Mobile oceanic species such as tuna can be captured by travelling long distances in large fishing vessels. A simpler alternative is to leverage off the fascination fish have with floating objects. When fishermen use such objects, they are called fish aggregating devices (FADs). FADs are anchored rafts or objects of any type, floating on the surface or just below it. Fishermen in the Pacific and Indian oceans set up floating FADs, assembled from all sorts of debris, around tropical islands, and then use purse seines to capture the fish attracted to them.[13]
A study using sonar in French Polynesia, found large shoals of juvenile bigeye tuna and yellowfin tuna aggregated closest to the devices, 10 to 50 m. Further out, 50 to 150 m, was a less dense group of larger yellowfin and albacore tuna. Yet further out, to 500 m, was a dispersed group of various large adult tuna. The distribution and density of these groups was variable and overlapped. The FADs were also used by other fish, and the aggregations dispersed when it was dark.[14]
Larger fish, even predator fish such as the great barracuda in the photo on the left, often attract a retinue of small fish that accompany them in a strategically safe way. Skindivers who remain for long periods in the water, also often attract a retinue of fish, with smaller fishes coming in close, and larger fishes observing from a greater distance. Marine turtles, functioning as a mobile shelter for small fish, can be impaled accidentally by a swordfish trying to catch the fish.[15]
Coastal fish

Coastal fish (also called neritic or inshore fish) inhabit the waters near the coast and above the continental shelf. Since the continental shelf is usually less than 200 metres deep, it follows that coastal fish that are not demersal fish are usually epipelagic fish, inhabiting the sunlit epipelagic zone.[2]
Coastal epipelagic fish are among the most abundant in the world. They include forage fish as well as the predator fish that feed on them. Forage fish thrive in those inshore waters where high productivity results from the upwelling and shoreline run off of nutrients. Some are partial residents that spawn in streams, estuaries and bays, but most complete their life cycle in the zone.[7]
Oceanic fish

Oceanic fish (also called open ocean or offshore fish) live in the waters that are not above the continental shelf. Oceanic fish can be contrasted with coastal fish, who do live above the continental shelf. However, the two types are not mutually exclusive, since there are no firm boundaries between coastal and ocean regions, and many epipelagic fish move between coastal and oceanic waters, particularly in different stages in their life cycle.[7]
Oceanic epipelagic fish can be true residents, partial residents, or accidental residents. True residents live their entire life in the open ocean. Only a few species are true residents, such as tuna, billfish, flying fish, sauries, pilotfish and remoras, dolphin, ocean sharks and ocean sunfish. Most of these species migrate back and forth across open oceans, rarely venturing over continental shelves. Some true residents associate with drifting jellyfish or seaweeds.[7]
Partial residents occur in three groups: species which live in the zone only when they are juveniles (drifting with jellyfish and seaweeds); species which live in the zone only when they are adults (salmon, flying fish, dolphin and whale sharks); and deep water species which make nightly migrations up into the surface waters (such as the lanternfish).[7] Accidental residents occur occasionally when adults and juveniles of species from other environments are carried by accident into the zone by currents.[7]
-

The huge ocean sunfish, a true resident of the ocean epipelagic zone, sometimes drifts with the current, eating jellyfish
-

The giant whale shark, another resident of the ocean epipelagic zone, filter feeds on plankton, and periodically dives deep into the mesopelagic zone.
-
.gif)
Lanternfish are partial residents of the ocean epipelagic zone. During the day they hide in deep waters, but at night they migrate up to surface waters to feed.
Deep water fish
In the deep ocean, the waters extend far below the epipelagic zone, and support very different types of pelagic fishes adapted to living in these deeper zones.[2]
In deep water, marine snow is a continuous shower of mostly organic detritus falling from the upper layers of the water column. Its origin lies in activities within the productive photic zone. Marine snow includes dead or dying plankton, protists (diatoms), fecal matter, sand, soot and other inorganic dust. The "snowflakes" grow over time and may reach several centimetres in diameter, travelling for weeks before reaching the ocean floor. However, most organic components of marine snow are consumed by microbes, zooplankton and other filter-feeding animals within the first 1,000 metres of their journey, that is, within the epipelagic zone. In this way marine snow may be considered the foundation of deep-sea mesopelagic and benthic ecosystems: As sunlight cannot reach them, deep-sea organisms rely heavily on marine snow as an energy source.
Some deep-sea pelagic groups, such as the lanternfish, ridgehead, marine hatchetfish, and lightfish families are sometimes termed pseudoceanic because, rather than having an even distribution in open water, they occur in significantly higher abundances around structural oases, notably seamounts and over continental slopes. The phenomenon is explained by the likewise abundance of prey species which are also attracted to the structures.
The fish in the different pelagic and deep water benthic zones are physically structured, and behave in ways, that differ markedly from each other. Groups of coexisting species within each zone all seem to operate in similar ways, such as the small mesopelagic vertically migrating plankton-feeders, the bathypelagic anglerfishes, and the deep water benthic rattails. "[16]
Ray finned species, with spiny fins, are rare among deep sea fishes, which suggests that deep sea fish are ancient and so well adapted to their environment that invasions by more modern fishes have been unsuccessful.[17] The few ray fins that do exist are mainly in the Beryciformes and Lampriformes, which are also ancient forms. Most deep sea pelagic fishes belong to their own orders, suggesting a long evolution in deep sea environments. In contrast, deep water benthic species, are in orders that include many related shallow water fishes.[18]
| Species by pelagic zone | |
|---|---|
| Many species move daily between zones in vertical migrations. In this table they are listed in the middle or deeper zone where they are regularly found. | |
| Zone | Species and species groups include: |
| Epipelagic[5] | |
| Mesopelagic | Lanternfish, opah, longnose lancetfish, barreleye, ridgehead, sabretooth, stoplight loosejaw, marine hatchetfish[19] |
| Bathypelagic | Principally bristlemouth and anglerfish. Also fangtooth, viperfish, black swallower, telescopefish, hammerjaw, daggertooth, barracudina, black scabbardfish, bobtail snipe eel, unicorn crestfish, gulper eel, flabby whalefish. |
| Benthopelagic[5] | Rattail and brotula are particularly abundant. |
| Benthic | Flatfish, hagfish, eelpout, greeneye eel, stingray, lumpfish, and batfish[5] |
| Comparative structure of pelagic fishes | ||||
|---|---|---|---|---|
| Epipelagic | Mesopelagic | Bathypelagic | deep sea benthic | |
| muscles | muscular bodies, ossified bones, scales, well developed gills and central nervous systems, and large hearts and kidneys. | poorly developed, flabby | ||
| skeleton | strong, ossified bones | weak, minimal ossification | ||
| scales | yes | none | ||
| nervous systems | well developed | lateral line and olfactory only | ||
| eyes | large and sensitive | small and may not function | variable (well developed to absent) | |
| photophores | absent | common | common | usually absent |
| gills | well developed | |||
| kidneys | large | small | ||
| heart | large | small | ||
| swimbladder | vertically migratory fish have swimbladders | reduced or absent | variable (well developed to absent) | |
| size | usually under 25 cm | variable, species greater than one metre are not uncommon | ||
Mesopelagic fish


Below the epipelagic zone, conditions change rapidly. Between 200 metres and about 1000 metres, light continues to fade until there is almost none. Temperatures fall through a thermocline to temperatures between 4 °C (39 °F) and 8 °C (46 °F). This is the twilight or mesopelagic zone. Pressure continues to increase, at the rate of one atmosphere every 10 metres, while nutrient concentrations fall, along with dissolved oxygen and the rate at which the water circulates."[2]
Sonar operators, using the newly developed sonar technology during World War II, were puzzled by what appeared to be a false sea floor 300–500 metres deep at day, and less deep at night. This turned out to be due to millions of marine organisms, most particularly small mesopelagic fish, with swimbladders that reflected the sonar. These organisms migrate up into shallower water at dusk to feed on plankton. The layer is deeper when the moon is out, and can become shallower when clouds pass over the moon. This phenomenon has come to be known as the deep scattering layer.[20]
Most mesopelagic fish make daily vertical migrations, moving at night into the epipelagic zone, often following similar migrations of zooplankton, and returning to the depths for safety during the day.[2][21] These vertical migrations often occur over a large vertical distances, and are undertaken with the assistance of a swimbladder. The swimbladder is inflated when the fish wants to move up, and, given the high pressures in the messoplegic zone, this requires significant energy. As the fish ascends, the pressure in the swimbladder must adjust to prevent it from bursting. When the fish wants to return to the depths, the swimbladder is deflated.[22] Some mesopelagic fishes make daily migrations through the thermocline, where the temperature changes between 10 and 20 °C, thus displaying considerable tolerances for temperature change.[23]
These fish have muscular bodies, ossified bones, scales, well developed gills and central nervous systems, and large hearts and kidneys. Mesopelagic plankton feeders have small mouths with fine gill rakers, while the piscivores have larger mouths and coarser gill rakers.[2] The vertically migratory fish have swimbladders.[17]
Mesopelagic fish are adapted for an active life under low light conditions. Most of them are visual predators with large eyes. Some of the deeper water fish have tubular eyes with big lenses and only rod cells that look upwards. These give binocular vision and great sensitivity to small light signals.[2] This adaptation gives improved terminal vision at the expense of lateral vision, and allows the predator to pick out squid, cuttlefish, and smaller fish that are silhouetted against the gloom above them.
Mesopelagic fish usually lack defensive spines, and use colour to camouflage them from other fish. Ambush predators are dark, black or red. Since the longer, red, wavelengths of light do not reach the deep sea, red effectively functions the same as black. Migratory forms use countershaded silvery colours. On their bellies, they often display photophores producing low grade light. For a predator from below, looking upwards, this bioluminescence camouflages the silhouette of the fish. However, some of these predators have yellow lenses that filter the (red deficient) ambient light, leaving the bioluminescence visible.[24]
-

The Antarctic toothfish have large, upward looking eyes, adapted to detecting the silhouettes of prey fish.[1]
-

The stoplight loosejaw has a lower jaw one-quarter as long as its body. The jaw has no floor and is attached only by a hinge and a modified tongue bone. Large fang-like teeth in the front are followed by many small barbed teeth.[3][4]
-
The stoplight loosejaw is also one of the few fishes that produce red bioluminescence. As most of their prey cannot perceive red light, this allows it to hunt with an essentially invisible beam of light.[3]
- ^ Froese, Rainer and Pauly, Daniel, eds. (2009). "Dissostichus mawsoni" in FishBase. August 2009 version.
- ^ Mystery Of Deep-sea Fish With Tubular Eyes And Transparent Head Solved ScienceDaily, 24 February 2009.
- ^ a b Kenaley, C.P (2007). "Revision of the Stoplight Loosejaw Genus Malacosteus (Teleostei: Stomiidae: Malacosteinae), with Description of a New Species from the Temperate Southern Hemisphere and Indian Ocean". Copeia 2007 (4): 886–900. doi:10.1643/0045-8511(2007)7[886:ROTSLG]2.0.CO;2.
- ^ Sutton, T.T. (Nov 2005). "Trophic ecology of the deep-sea fish Malacosteus niger (Pisces: Stomiidae): An enigmatic feeding ecology to facilitate a unique visual system?". Deep Sea Research Part I: Oceanographic Research Papers 52 (11): 2065–2076. doi:10.1016/j.dsr.2005.06.011.
The brownsnout spookfish is a species of barreleye is the only vertebrate known to employ a mirror, as opposed to a lens, to focus an image in its eyes.[25][26]
Sampling via deep trawling indicates that lanternfish account for as much as 65% of all deep sea fish biomass.[27] Indeed, lanternfish are among the most widely distributed, populous, and diverse of all vertebrates, playing an important ecological role as prey for larger organisms. The estimated global biomass of lanternfish is 550–660 million metric tonnes, several times the entire world fisheries catch. Lanternfish also account for much of the biomass responsible for the deep scattering layer of the world's oceans. Sonar reflects off the millions of lanternfish swim bladders, giving the appearance of a false bottom.[28]
Bigeye tuna are an epipelagic/mesopelagic species that eats other fish. Satellite tagging has shown that bigeye tuna often spend prolonged periods cruising deep below the surface during the daytime, sometimes making dives as deep as 500 metres. These movements are thought to be in response to the vertical migrations of prey organisms in the deep scattering layer.
-

Longnose lancetfish. Lancetfish are ambush predators which spend all their time in the mesopelagic zone. They are among the largest mesopelagic fishes (up to 2 metres).[1]
-

The telescopefish has large, forward-pointing telescoping eyes with large lenses.[2]
-
The daggertooth paralyses other mesopelagic fish when it bites them with its dagger-like teeth.[3]
- ^ Moyle and Cech, p. 336
- ^ Froese, Rainer and Pauly, Daniel, eds. (2010). "Gigantura chuni" in FishBase. October 2010 version.
- ^ Froese, Rainer and Pauly, Daniel, eds. (2010). "Anotopterus pharao" in FishBase. April 2010 version.
Bathypelagic fish


Below the mesopelagic zone it is pitch dark. This is the midnight or bathypelagic zone, extending from 1000 m to the bottom deep water benthic zone. If the water is exceptionally deep, the pelagic zone below 4000 m is sometimes called the lower midnight or abyssopelagic zone.
Conditions are somewhat uniform throughout these zones, the darkness is complete, the pressure is crushing, and temperatures, nutrients and dissolved oxygen levels are all low.[2]
Bathypelagic fish have special adaptations to cope with these conditions – they have slow metabolisms and unspecialized diets, being willing to eat anything that comes along. They prefer to sit and wait for food rather than waste energy searching for it. The behaviour of bathypelagic fish can be contrasted with the behaviour of mesopelagic fish. Mesopelagic are often highly mobile, whereas bathypelagic fish are almost all lie-in-wait predators, normally expending little energy in movement.[33]
The dominant bathypelagic fishes are small bristlemouth and anglerfish; fangtooth, viperfish, daggertooth and barracudina are also common. These fishes are small, many about 10 centimetres long, and not many longer than 25 cm. They spend most of their time waiting patiently in the water column for prey to appear or to be lured by their phosphors. What little energy is available in the bathypelagic zone filters from above in the form of detritus, faecal material, and the occasional invertebrate or mesopelagic fish.[33] About 20% of the food that has its origins in the epipelagic zone falls down to the mesopelagic zone,[20] but only about 5% filters down to the bathypelagic zone.[29]
Bathypelagic fish are sedentary, adapted to outputting minimum energy in a habitat with very little food or available energy, not even sunlight, only bioluminescence. Their bodies are elongated with weak, watery muscles and skeletal structures. Since so much of the fish is water, they are not compressed by the great pressures at these depths. They often have extensible, hinged jaws with recurved teeth. They are slimy, without scales. The central nervous system is confined to the lateral line and olfactory systems, the eyes are small and may not function, and gills, kidneys and hearts, and swimbladders are small or missing.[29][34]
These are the same features found in fish larvae, which suggests that during their evolution, bathypelagic fish have acquired these features through neoteny. As with larvae, these features allow the fish to remain suspended in the water with little expenditure of energy.[35]
Despite their ferocious appearance, these beasts of the deep are mostly miniature fish with weak muscles, and are too small to represent any threat to humans.
The swimbladders of deep sea fish are either absent or scarcely operational, and bathypelagic fish do not normally undertake vertical migrations. Filling bladders at such great pressures incurs huge energy costs. Some deep sea fishes have swimbladders which function while they are young and inhabit the upper epipelagic zone, but they wither or fill with fat when the fish move down to their adult habitat.[36]
The most important sensory systems are usually the inner ear, which responds to sound, and the lateral line, which responds to changes in water pressure. The olfactory system can also be important for males who find females by smell.[37] Bathypelagic fish are black, or sometimes red, with few photophores. When photophores are used, it is usually to entice prey or attract a mate. Because food is so scarce, bathypelagic predators are not selective in their feeding habits, but grab whatever come close enough. They accomplish this by having a large mouth with sharp teeth for grabbing large prey and overlapping gill rakers which prevent small prey that have been swallowed from escaping.[34]
It is not easy finding a mate in this zone. Some species depend on bioluminescence. Others are hermaphrodites, which doubles their chances of producing both eggs and sperm when an encounter occurs.[29] The female anglerfish releases pheromones to attract tiny males. When a male finds her, he bites on to her and never lets go. When a male of the anglerfish species Haplophryne mollis bites into the skin of a female, he release an enzyme that digests the skin of his mouth and her body, fusing the pair to the point where the two circulatory systems join up. The male then atrophies into nothing more than a pair of gonads. This extreme sexual dimorphism ensures that, when the female is ready to spawn, she has a mate immediately available.[38]
Many forms other than fish live in the bathypelagic zone, such as squid, large whales, octopuses, sponges, brachiopods, sea stars, and echinoids, but this zone is difficult for fish to live in.
-

The gulper eel uses its mouth like a net by opening its large mouth and swimming at its prey. It has a luminescent organ at the tip of its tail to attract prey.
-

The black swallower, with its distensible stomach, is notable for its ability to swallow, whole, bony fishes ten times its mass.[1][2]
-
.gif)
Female Haplophryne mollis anglerfish trailing attached males which have atrophied into a pair of gonads, for use when the female is ready to spawn.
-

The Sloane's viperfish can make nightly migrations from bathypelagic depths to near surface waters.[4]
- ^ Jordan, D.S. (1905). A Guide to the Study of Fishes. H. Holt and Company.
- ^ Froese, Rainer and Pauly, Daniel, eds. (2009). "Chiasmodon niger" in FishBase. August 2009 version.
- ^ Froese, Rainer and Pauly, Daniel, eds. (2009). "Anoplogaster cornuta" in FishBase. August 2009 version.
- ^ Froese, Rainer and Pauly, Daniel, eds. (2010). "Chauliodus sloani" in FishBase. April 2010 version.
Demersal fish

Demersal fish live on or near the bottom of the sea.[39] Demersal fish are found by the seafloor in coastal areas on the continental shelf, and in the open ocean they are found along the outer continental margin on the continental slope and the continental rise. They are not generally found at abyssopelagic or hadopelagic depths or on the abyssal plain. They occupy a range of seafloors consisting of mud, sand, gravel or rocks.[39]
In deep waters, the fishes of the demersal zone, compared to fishes of the bathypelagic zone, are active and relatively abundant."[33]
Rattails and brotulas are common, and other well-established families are eels, eelpouts, hagfishes, greeneyes, batfishes and lumpfishes.[34]
The bodies of deep water benthic fishes are muscular with well developed organs. In this way they are closer to mesopelagic fishes than bathopelagic fishes. In other ways, they are more variable. Photophores are usually absent, eyes and swimbladders range from absent to well developed. They vary in size, with larger species greater than one metre not uncommon.
Deep sea benthic fish are usually long and narrow. Many are eels or shaped like eels. This may be because long bodies have long lateral lines. Lateral lines detect low-frequency sounds, and some benthic fishes appear to have muscles that drum such sounds to attract mates.[17] Smell is also important, as indicated by the rapidity with which benthic fish find traps baited with bait fish.
The main diet of deep sea benthic fish is invertebrates of the deep sea benthos and carrion. Smell, touch and lateral line sensitivities seem to be the main sensory devices for locating these.[40]
Deep sea benthic fish can be divided into strictly benthic fish and benthopelagic fish. Usually strictly benthic fish are negatively buoyant while benthopelagic fish are neutrally buoyant. Strictly benthic fish stay in constant contact with the bottom. They either lie-and-wait as ambush predators or move actively over the bottom in search for food.[40]
Benthopelagic fish
Benthopelagic fish inhabit the water just above the bottom, feeding on benthos and benthopelagic zooplankton.[41] Most dermersal fish are benthopelagic.[39]
They can be divided into flabby or robust body types. Flabby benthopelagic fishes are like bathopelagic fishes, they have a reduced body mass, and low metabolic rates, expending minimal energy as they lie and wait to ambush prey.[42] An example of a flabby fish is the cusk-eel Acanthonus armatus,[43] a predator with a huge head and a body that is 90% water. This fish has the largest ears (otoliths) and the smallest brain in relation to its body size of all known vertebrates.[44]
Robust benthopelagic fish are muscular swimmers that actively cruise the bottom searching for prey. They may live around features, such as seamounts, which have strong currents.[44] Examples are the orange roughy and Patagonian toothfish. Because these fish were once abundant, and because their robust bodies are good to eat, these fish have been commercially harvested.[45][46]
Benthic fish
Benthic fish are not pelagic fish, but they are discussed here briefly, by way of completeness and contrast.
Some fishes don't fit into the above classification. For example, the family of nearly blind spiderfishes, common and widely distributed, feed on benthopelagic zooplankton. Yet they are strictly benthic fish, since they stay in contact with the bottom. Their fins have long rays they use to "stand" on the bottom while they face the current and grab zooplankton as it passes by.[47]
The deepest-living fish known, the strictly benthic Abyssobrotula galatheae, eel-like and blind, feeds on benthic invertebrates.[48][49]
-

Pacific hagfish resting on bottom. Hagfish coat themselves and any dead fish they find with noxious slime making them inedible to other species."
-

The tripodfish (Bathypterois grallator), a species of spiderfish, uses its fin extensions to "stand" on the bottom.[1]
-

The blotched fantail ray feeds on bottom-dwelling fish, bivalves, crabs and shrimps.[2]
- ^ Froese, Rainer and Pauly, Daniel, eds. (2009). "Bathypterois grallator" in FishBase. August 2009 version.
- ^ Froese, Rainer and Pauly, Daniel, eds. (2009). "Taeniura meyeni" in FishBase. August 2009 version.

At great depths, food scarcity and extreme pressure works to limit the survivability fish. The deepest point of the ocean is about 11,000 metres. Bathypelagic fishes are not normally found below 3,000 metres. The greatest depth recorded for a benthic fish is 8,370 m.[50] It may be that extreme pressures interfere with essential enzyme functions.[29]
Benthic fishes are likely to be found, and are more diverse, on the continental slope, where there is habitat diversity and often food supplies. About 40% of the ocean floor consists of abyssal plains, but these flat, featureless regions are covered with sediment and largely devoid of benthic life (benthos). Deep sea benthic fishes are more likely to associate with canyons or rock outcroppings among the plains, where invertebrate communities are established. Undersea mountains (seamounts) can intercept deep sea currents, and cause productive upwellings which support benthic fish. Undersea mountain ranges can separate underwater regions into different ecosystems.[16]
Pelagic fisheries
Forage fish
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Important marine wild fisheries |
Small pelagic fish are usually forage fish that are hunted by larger pelagic fish and other predators. Forage fish filter feed on plankton and are usually less than 10 centimetres long. They often stay together in schools and may migrate large distances between spawning grounds and feeding grounds. They are found particularly in upwelling regions around the northeast Atlantic, off the coast of Japan, and off the west coasts of Africa and the Americas. Forage fish are generally short-lived, and their stocks fluctuate markedly over the years. [51]
Herring are found in the North Sea and the North Atlantic at depths to 200 meters. Important herring fisheries have existed in these areas for centuries. Herring of different sizes and growth rates belong to different populations, each of which have their own migration routes. When spawning, a female produces from 20,000 to 50,000 eggs. After spawning, the herrings are depleted in fat, and migrate back to feeding grounds rich in plankton.[52] Around Iceland, three separate populations of herring were traditionally fished. These stocks collapsed in the late 1960s, although two have since recovered. After the collapse, Iceland turned to capelin, which now account for about half of Iceland's total catch.[53]
Blue whiting are found in the open ocean and above the continental slope at depths between 100 and 1000 meters. They follow vertical migrations of the zooplankton they feed on to the bottom during daytime and to the surface at night time.[52][54]
Traditional fisheries for anchovies and sardines have also operated in the Pacific, the Mediterranean, and the southeast Atlantic.[55] The world annual catch of forage fish in recent years has been around 22 million tonnes, or one quarter of the world's total catch.
-

These schooling Pacific sardines are forage fish
-

Peruvian anchoveta
Predator fish
Medium size pelagic fishes include trevally, barracuda, flying fish, bonito, mahi mahi and coastal mackerel.[1] Many of these fish hunt forage fish, but are in turn hunted by yet larger pelagic fish. Nearly all fish are predator fish to some measure, and apart from the top predators, the distinction between predator fish and prey or forage fish is somewhat artificial.[56]
Around Europe there are three populations of coastal mackerel. One population migrates to the North Sea, another stays in of the Irish Sea, and the third population migrates southwards along the west coast of Scotland and Ireland. The mackerel's cruise speed is an impressive 10 kilometres per hour.[52][57]
Many large pelagic fish are oceanic nomadic species which undertake long offshore migrations. They feed on small pelagic forage fish, as well as medium-sized pelagic fish. At times, they follow their schooling prey, and many species form schools themselves.
Examples of larger pelagic fish are tuna, billfish, king mackerel and sharks and large rays.
Tuna in particular are of major importance to commercial fisheries. Though tuna migrate across oceans, trying to find them there is not the usual approach. Tuna tend to congregate in areas where food is abundant, along the boundaries of currents, around islands, near seamounts, and in some areas of upwelling along continental slopes. Tuna are captured by several methods: purse seine vessels enclose an entire surface school with special nets, pole and line vessels which use poles baited with other smaller pelagic fish as baitfish, and rafts called fish aggregating devices are set up, because tuna, as well as some other pelagic fish, tend to congregate under floating objects.[1]
Other large pelagic fish are premier game fish, particularly marlin and swordfish.
-

Yellowfin tuna are being fished as a replacement for the now largely depleted Southern bluefin tuna.
-
King mackerels cruise on long migrations at 10 kilometres per hour.[1][2]
- ^ Cite error: The named reference
pfawas invoked but never defined (see the help page). - ^ Cite error: The named reference
Mackerelwas invoked but never defined (see the help page).



Productivity
Upwelling occurs both along coastlines and in midocean when a collision of deep ocean currents brings cold water rich in nutrients to the surface. These upwellings support blooms of phytoplankton, which in turn produce zooplankton and support many of the world's main fisheries. If the upwelling fails then fisheries in the area fail.[13]
In the 1960s the Peruvian anchoveta fishery was the world's largest fishery. The anchoveta population was greatly reduced during the 1972 El Niño event, when warm water drifted over the cold Humboldt Current, as part of a 50-year cycle, lowering the depth of the thermocline. The upwelling stopped and phytoplankton production plummeted, as did the anchoveta population, and millions of seabirds, dependent on the anchoveta, died.[58] Since the mid-1980s, the upwelling has resumed, and the Peruvian anchoveta catch levels have returned to the 1960s levels.
Off Japan, the collision of the Oyashio Current with the Kuroshio Current produces nutrient-rich upwellings. Cyclic changes in these currents resulted in a decline in the sardine sardinops melanosticta populations. Fisheries catches fell from 5 million tonnes in 1988 to 280 thousand tonnes in 1998. As a further consequence, Pacific bluefin tuna stopped moving into the region to feed.[59][60]
Ocean currents can shape how fish are distributed, both concentrating and dispersing them. Adjacent ocean currents can define distinct, if shifting, boundaries. These boundaries can even be visible, but usually their presence is marked by rapid changes in salinity, temperature and turbidity.[13]
For example, in the Asian northern Pacific, albacore are confined between two current systems. The northern boundary is determined by the cold North Pacific Current and the southern boundary is determined by the North Equatorial Current. To complicate things, their distribution is further modified within the area defined by the two current systems by another current, the Kuroshio Current, whose flows fluctuate seasonally.[61]
Epipelagic fish often spawn in an area where the eggs and larvae drift downstream into suitable feeding areas, and eventually drift into adult feeding areas.[13]
Islands and banks can interact with currents and upwellings in a manner that results in areas of high ocean productivity. Large eddies can form downcurrent or downwind from islands, concentrating plankton.[62] Banks and reefs can intercept deep currents that upwell.[13]
Highly migratory species

Epipelagic fish generally move long distances between feeding and spawning areas, or as a response to changes in the ocean. Large ocean predators, such as salmon and tuna, can migrate thousands of kilometres, crossing oceans.[64]
In a 2001 study, the movements of Atlantic bluefin tuna from an area off North Carolina were studied with the help of special popup tags. When attached to a tuna, these tags monitored the movements of the tuna for about a year, then freed themselves, and floated to the surface where they transmitted their information to a satellite. The study found that the tuna had four different migration patterns. One group confined itself to the western Atlantic for a year. Another group also stayed mainly in the western Atlantic, but migrated to the Gulf of Mexico for spawning. A third group moved across the Atlantic ocean and back again. The fourth group crossed to the eastern Atlantic and then moved into the Mediterranean Sea for spawning. The study indicates that, while there is some differentiation by spawning areas, there is essentially only one population of Atlantic bluefin tuna, intermixing groups that between them use all of the north Atlantic ocean, the Gulf of Mexico and the Mediterranean Sea.[65]
The term highly migratory species (HMS) is a legal term which has its origins in Article 64 of the United Nations Convention on the Law of the Sea (UNCLOS).[66]
The highly migratory species include: tuna and tuna-like species (albacore, Atlantic bluefin, bigeye tuna, skipjack, yellowfin, blackfin, little tunny, Pacific bluefin, southern bluefin and bullet), pomfret, marlin, sailfish, swordfish, saury and oceangoing sharks, dolphins and other cetaceans.
Essentially, highly migratory species coincide with the larger of the "large pelagic fish", discussed in the previous section, if cetaceans are added and some commercially unimportant fish, such as the sunfish, are excluded. These are high trophic level species which undertake migrations of significant but variable distances across oceans for feeding, often on forage fish, or reproduction, and also have wide geographic distributions. Thus, these species are found both inside the 200-nautical-mile (370 km) exclusive economic zones and in the high seas outside these zones. They are pelagic species, which means they mostly live in the open ocean and do not live near the sea floor, although they may spend part of their life cycle in nearshore waters.[67]
Capture production
According to the Food and Agriculture Organization (FAO), the world harvest in 2005 consisted of 93.2 million tonnes captured by commercial fishing in wild fisheries.[68] Of this total, about 45% were pelagic fish. The following table shows the world capture production in tonnes.[69]
| Capture production by groups of species in tonnes | ||||||||
|---|---|---|---|---|---|---|---|---|
| Type | Group | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 |
| Small pelagic fish | Herrings, sardines, anchovies | 22 671 427 | 24 919 239 | 20 640 734 | 22 289 332 | 18 840 389 | 23 047 541 | 22 404 769 |
| Large pelagic fish | Tunas, bonitos, billfishes | 5 943 593 | 5 816 647 | 5 782 841 | 6 138 999 | 6 197 087 | 6 160 868 | 6 243 122 |
| Other pelagic fish | 10 712 994 | 10 654 041 | 12 332 170 | 11 772 320 | 11 525 390 | 11 181 871 | 11 179 641 | |
| Cartilaginous fish | Sharks, rays, chimaeras | 858 007 | 870 455 | 845 854 | 845 820 | 880 785 | 819 012 | 771 105 |
Threatened species
In 2009, the International Union for Conservation of Nature (IUCN) produced the first red list for threatened oceanic sharks and rays. They claim that about one third of open ocean sharks and rays are under threat of extinction.[70] There are 64 species of oceanic sharks and rays on the list, including hammerheads, giant devil rays and porbeagle.[71]
Oceanic sharks are captured incidentally by swordfish and tuna high seas fisheries. In the past there were few markets for sharks, which were regarded as worthless bycatch. Now sharks are being increasingly targeted to supply emerging Asian markets, particularly for shark fins, which are used in shark fin soup.[71]
The northwest Atlantic Ocean shark populations are estimated to have declined by 50% since the early 1970s. Oceanic sharks are vulnerable because they don't produce many young, and the young can take decades to mature.[71]
-

The scalloped hammerhead is classified as endangered
-

The oceanic whitetip shark has declined by 99% in the Gulf of Mexico[1]
-

The devil fish, a large ray, is also threatened
-

So is the porbeagle shark
- ^ Cite error: The named reference
guardianwas invoked but never defined (see the help page).
In parts of the world the scalloped hammerhead shark has declined by 99% since the late 1970s. Its status on the red list is that it is globally endangered, meaning it is near extinction.[71]
See also
References
Notes
- 1 2 3 4 5 Lal, Brij V.; Fortune, Kate (2000). The Pacific Islands: An Encyclopedia. University of Hawaii Press. p. 8. ISBN 978-0-8248-2265-1.
- 1 2 3 4 5 6 7 8 Moyle and Cech, p. 585
- ↑ McLintock, A H (ed.) "Fish, Marine". Te Ara – The Encyclopaedia of New Zealand. Updated 18 September 2007.
- ↑ Walrond, Carl. "Oceanic fish". Encyclopedia of New Zealand. Updated 21 September 2007.
- 1 2 3 4 5 6 7 Moyle and Cech, p. 571
- 1 2 Herring, Peter (2002) The Biology of the Deep Ocean, pp. 192–195, Oxford University Press. ISBN 978-0-19-854956-7.
- 1 2 3 4 5 6 7 Moyle and Cech, p. 572
- ↑ Blackburn (1965). "Oceanography and the ecology of tunas". Oceanography and Marine Biology: An Annual Review 3: 299–322.
- ↑ Hunter, JR; Mitchell CT (1966). "Association of fishes with flotsam in the offshore waters of Central America". US Fishery Bulletin 66: 13–29.
- ↑ Kingsford MJ (1993). "Biotic and abiotic structure in the pelagic environment: Importance to small fishes". Bulletin of Marine Science 53 (2): 393–415.
- ↑ Dooley JK (1972). "Fishes associated with the pelagic sargassum complex, with a discussion of the sargassum community". Contributions in Marine Science 16: 1–32.
- ↑ Moyle and Cech, p. 576
- 1 2 3 4 5 Moyle and Cech, pp. 574–575
- ↑ Josse, E. (2000). "Typologie et comportement des agrégations thonières autour de dispositifs de concentration de poissons à partir de prospections acoustiques en Polynésie française". Aquatic Living Resources 13 (4): 183–192. doi:10.1016/S0990-7440(00)00051-6.
- ↑ Frazier, J. G.; Fierstine, H. L.; Beavers, S. C.; Achaval, F.; Suganuma, H.; Pitman, R. L.; Yamaguchi, Y.; Prigioni, C. M. (1994). "Impalement of marine turtles (Reptitia, Chelonia: Cheloniidae and Dermochelyidae) by billfishes (Osteichthyes, Perciformes: Istiophoridae and Xiphiidae)". Environmental Biology of Fishes 39: 85–96. doi:10.1007/BF00004759.
- 1 2 Moyle and Cech, p. 591
- 1 2 3 Haedrich, R. L. (1996). "Deep-water fishes: Evolution and adaptation in the earth's largest living spaces". Journal of Fish Biology 49: 40–53. doi:10.1111/j.1095-8649.1996.tb06066.x.
- ↑ Moyle and Cech, p. 586
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2009). "Argyropelecus aculeatus" in FishBase. August 2009 version.
- 1 2 Ryan P "Deep-sea creatures: The mesopelagic zone" Te Ara – the Encyclopedia of New Zealand. Updated 21 September 2007.
- ↑ Bone and Moore, p. 38.
- ↑ Douglas, E.; Friedl, W.; Pickwell, G. (1976). "Fishes in oxygen-minimum zones: Blood oxygenation characteristics". Science 191 (4230): 957–9. Bibcode:1976Sci...191..957D. doi:10.1126/science.1251208. PMID 1251208.
- ↑ Moyle and Cech, p. 590
- ↑ Muntz, W. R. A. (2009). "On yellow lenses in mesopelagic animals". Journal of the Marine Biological Association of the United Kingdom 56 (4): 963. doi:10.1017/S0025315400021019.
- ↑ Wagner, H.J., Douglas, R.H., Frank, T.M., Roberts, N.W., and Partridge, J.C. (27 January 2009). "A Novel Vertebrate Eye Using Both Refractive and Reflective Optics". Current Biology 19 (2): 108–114. doi:10.1016/j.cub.2008.11.061. PMID 19110427.
- ↑ Smith, L. (8 January 2009). "Fish with four eyes can see through the deep sea gloom". Times Online. Times Newspapers Ltd. Retrieved 14 March 2009.
- ↑ Hulley, P. Alexander (1998). Paxton, J.R. & Eschmeyer, W.N., ed. Encyclopedia of Fishes. San Diego: Academic Press. pp. 127–128. ISBN 0-12-547665-5.
- ↑ Cornejo, R.; Koppelmann, R. and Sutton, T. (2006). "Deep-sea fish diversity and ecology in the benthic boundary layer".
- 1 2 3 4 5 Ryan P "Deep-sea creatures: The bathypelagic zone" Te Ara – the Encyclopedia of New Zealand. Updated 21 September 2007.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2006). "Gonostoma bathyphilum" in FishBase. January 2006 version.
- ↑ Froese, Rainer, and Daniel Pauly, eds. (2009). "Gonostoma" in FishBase. August 2009 version.
- ↑ Schmid, Randolph E. (22 January 2009). "Scientists solve mystery: 3 fish are all the same". Associated Press.
- 1 2 3 Moyle and Cech, p. 594
- 1 2 3 Moyle and Cech, p. 587
- ↑ Marshall (1984) "Progenetic tendencies in deep-sea fishes", pp. 91–101 in Potts GW and Wootton RJ (eds.) (1984) Fish reproduction: strategies and tactics Fisheries Society of the British Isles.
- ↑ Horn MH (1970). "The swimbladder as a juvenile organ in stromateoid fishes". Breviora 359: 1–9.
- ↑ Jumper, J.; Baird, R. C. (1991). "Location by Olfaction: A Model and Application to the Mating Problem in the Deep-Sea Hatchetfish Argyropelecus hemigymnus". The American Naturalist 138 (6): 1431. doi:10.1086/285295. JSTOR 2462555.
- ↑ Pietsch, T. W. (1975). "Precocious sexual parasitism in the deep sea ceratioid anglerfish, Cryptopsaras couesi Gill". Nature 256 (5512): 38–40. Bibcode:1975Natur.256...38P. doi:10.1038/256038a0.
- 1 2 3 Walrond C Carl . "Coastal fish – Fish of the open sea floor" Te Ara – the Encyclopedia of New Zealand. Updated 2 March 2009
- 1 2 Moyle and Cech, p. 588
- ↑ Mauchline J; Gordon JDM (1986). "Foraging strategies of deep-sea fish" (PDF). Mar. Ecol. Prog. Ser. 27: 227–238. doi:10.3354/meps027227.
- ↑ Koslow, J. A. (1996). "Energetic and life-history patterns of deep-sea benthic, benthopelagic and seamount-associated fish". Journal of Fish Biology 49: 54–74. doi:10.1111/j.1095-8649.1996.tb06067.x.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2009). "Acanthonus armatus" in FishBase. August 2009 version.
- 1 2 Fine, M. L.; Horn, M. H.; Cox, B. (1987). "Acanthonus armatus, a Deep-Sea Teleost Fish with a Minute Brain and Large Ears". Proceedings of the Royal Society B: Biological Sciences 230 (1259): 257–65. Bibcode:1987RSPSB.230..257F. doi:10.1098/rspb.1987.0018. JSTOR 36061. PMID 2884671.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2009). "Hoplostethus atlanticus" in FishBase. August 2009 version.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2009). "Dissostichus eleginoides" in FishBase. August 2009 version.
- ↑ Sulak KJ. "The systematics and biology of Bathypterois (Pisces, Chlorophthalmidae) with a revised classification of benthic myctophiform fishes". Ichthyological Research 32 (4): 443–446.
- ↑ Nielsen JG (1977). "The deepest living fish Abyssobrotula galatheae: a new genus and species of oviparous ophidioids (Pisces, Brotulidae)". Galathea Report 14: 41–48.
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2009). "Abyssobrotula galatheae" in FishBase. August 2009 version.
- ↑ Nielsen, J.G. (1977). "The deepest living fish Abyssobrotula galatheae: a new genus and species of oviparous ophidioids (Pisces, Brotulidae)". Galathea Report 14: 41–48.
- ↑ Checkley D, Alheit J and Oozeki Y (2009) Climate Change and Small Pelagic Fish, Cambridge University Press. ISBN 0-521-88482-9.
- 1 2 3 Pelagic species Pelagic Freezer-trawler Association. Retrieved 22 July 2009.
- ↑ Pelagic fishes Icelandic fisheries. Retrieved 24 July 2009.
- ↑ Blue whiting Institute of Marine Research. Retrieved 23 July 2009.
- ↑ Bone and Moore, p. 443
- ↑ FAO: LAPE project Forage species Rome. Updated 28 November 2008.
- ↑ Mackerel Institute of Marine Research. Retrieved 23 July 2009.
- ↑ Chavez, F. P.; Ryan, John; Lluch-Cota, Salvador E.; Ñiquen c., Miguel (2003). "From Anchovies to Sardines and Back: Multidecadal Change in the Pacific Ocean". Science 299 (5604): 217–21. Bibcode:2003Sci...299..217C. doi:10.1126/science.1075880. PMID 12522241.
- ↑ Polovina, J. J. (1996). "Decadal variation in the trans-Pacific migration of northern bluefin tuna (Thunnus thynnus) coherent with climate-induced change in prey abundance". Fisheries Oceanography 5 (2): 114–119. doi:10.1111/j.1365-2419.1996.tb00110.x.
- ↑ FAO: Species Fact Sheets: Sardinops melanostictus (Schlegel, 1846) Rome. Retrieved 18 August 2009.
- ↑ Nakamura, Hiroshi (1969). Tuna distribution and migration. Fishing News.
- ↑ Blackburn M (1965). "Oceanography and the ecology of tunas" (PDF). Oceanography and Marine Biology Annual Revue 3: 299–322.
- ↑ Casey, J. G.; Kohler, N. E. (1992). "Tagging studies on the Shortfin Mako Shark (Isurus oxyrinchus) in the Western North Atlantic". Marine and Freshwater Research 43: 45. doi:10.1071/MF9920045.
- ↑ Moyle and Cech, p. 578
- ↑ Block, B. A.; Dewar, H; Blackwell, S. B.; Williams, T. D.; Prince, E. D.; Farwell, C. J.; Boustany, A; Teo, S. L.; Seitz, A; Walli, A; Fudge, D (2001). "Migratory Movements, Depth Preferences, and Thermal Biology of Atlantic Bluefin Tuna" (PDF). Science 293 (5533): 1310–4. Bibcode:2001Sci...293.1310B. doi:10.1126/science.1061197. PMID 11509729.
- ↑ United Nations Convention on the Law of the Sea: Text
- ↑ Pacific Fishery Management Council: Background: Highly Migratory Species
- ↑ Fisheries and Aquaculture. FAO. Retrieved on 2015-05-01.
- ↑ FAO (2007) State of the World Fisheries and Aquaculture 2006. Fisheries and Aquaculture Department. ISBN 978-92-5-105568-7
- ↑ Third of open ocean sharks threatened with extinction IUCN. 25 June 2009.
- 1 2 3 4 Fishing puts a third of all oceanic shark species at risk of extinction guardian.co.uk, 26 June 2009.
Bibliography
- Bone, Quentin; Moore, Richard (2008). Biology of Fishes. Garland Science. ISBN 978-0-203-88522-2.
- Moyle, PB and Cech, JJ (2004). Fishes, An Introduction to Ichthyology (5th ed.). Benjamin Cummings. ISBN 978-0-13-100847-2.
Further reading
- Collette, BB (2010) "Reproduction and development in epipelagic fishes" In: Kathleen S Cole, Reproduction and Sexuality in Marine Fishes: Patterns and Processes, pp. 21–64, University of California Press. ISBN 978-0-520-26433-5.
- Freon, Pierre (1998) Dynamics of Pelagic Fish Distribution and Behaviour: Effects on Fisheries and Stock Assessment, Wiley-Blackwell. ISBN 978-0-85238-241-7.
- Johnsen, S (2003). "Lifting the Cloak of Invisibility: The Effects of Changing Optical Conditions on Pelagic Crypsis1". Integrative and Comparative Biology 43 (4): 580–590. doi:10.1093/icb/43.4.580.
- Makris, N; Ratilal, P; Jagannathan, S; Gong, Z; Andrews, M; Bertsatos, I; Godo, OR; Nero, RW; Jech, JM (2009). "Critical Population Density Triggers Rapid Formation of Vast Oceanic Fish Shoals". Science 323: 1734–1737. doi:10.1126/science.1169441.
- Pepperell J (2011) Fishes of the Open Ocean: A Natural History and Illustrated Guide University of New South Wales Press, ISBN 978-1-74223-267-6.
- Salvanesa AGV and Kristoffersen JB "Mesopelagic Fishes" In: Encyclopedia of Ocean Sciences, pp. 1711–1717. doi:10.1006/rwos.2001.0012
- Scientists IDs genesis of animal behavior patterns PhysOrg.com, 26 March 2009.
- One fish, two fish: New MIT sensor improves fish counts PhysOrg.com, 2 February 2006.
External links
| Wikimedia Commons has media related to Deep sea fish. |
- Pelagic fish – Institute of Marine Research
- Glowing life in an underwater world TED video from Edith Widder
- The Open Ocean MarineBio.org. MarineBio.org. Updated 28 August 2011. TED video from Edith Widder
- The Open Ocean MarineBio.org. MarineBio.org. Updated 28 August 2011.
- Pelagic Regional Advisory Council of the European Commission
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||











